Alemtuzumab by continuous intravenous infusion followed by subcutaneous injection plus rituximab in the treatment of patients with chronic lymphocytic leukemia recurrence
- PMID: 20225334
- PMCID: PMC4476388
- DOI: 10.1002/cncr.24958
Alemtuzumab by continuous intravenous infusion followed by subcutaneous injection plus rituximab in the treatment of patients with chronic lymphocytic leukemia recurrence
Erratum in
- Cancer. 2010 Aug 15;116(16):3982. Dosage error in article text
Abstract
Background: Monoclonal antibodies may be used more effectively in combination. A previous study of intravenous (iv) bolus alemtuzumab plus rituximab in patients with chronic lymphocytic leukemia (CLL) recurrence produced a response rate of 54% after a 4-week treatment period.
Methods: To optimize dose, schedule, and route of alemtuzumab, a study was designed exploring continuous intravenous infusion (civ) followed by subcutaneous (sc) alemtuzumab together with weekly iv rituximab in patients with previously treated CLL.
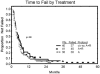
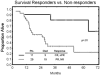
Results: Data from 40 patients with a median age of 59 years, and a median of 3 prior regimens (range, 1-8 regimens) were evaluable. Approximately 64% of patients were fludarabine-refractory. Seven patients (18%) achieved a complete response (CR), 4 (10%) a nodular partial response (nPR), and 10 (25%) a partial response for an overall response rate of 53%. Of 11 major responses (CR, nPR), 8 occurred after cycle 1. Response rates were highest in blood (94%), followed by liver/spleen (82%), bone marrow (68%), and lymph nodes (51%). The combination did not generate unexpected toxicities. Cytomegalovirus (CMV) reactivations occurred in 6 patients (15%) and responded well to anti-CMV therapy. High titers of anti-idiotype antibodies after sc alemtuzumab were demonstrated in 1 patient, but remained without clinical sequelae.
Conclusions: The combination of civ/sc alemtuzumab plus rituximab has activity in some patients with recurrent/refractory CLL and maximum response is achieved after 1 cycle (4 weeks) in 73% of patients. Further exploration in other settings of CLL together with accompanying pharmacokinetic studies is recommended.
(c) 2010 American Cancer Society.
Conflict of interest statement
Figures
References
-
- Dighiero G, Hamblin TJ. Chronic lymphocytic leukemia. Lancet. 2008;371:1017–1029. - PubMed
-
- O'Brien S. New agents in the treatment of CLL. Hematology Am Soc Hematol Educ Program. 2008:457–464. - PubMed
-
- Faderl S, Thomas DA, O'Brien S, et al. Experience with alemtuzumab plus rituximab in patients with relapsed and refractory lymphoid malignancies. Blood. 2003;101:3413–3415. - PubMed
-
- Ferrajoli A, Wierda W, LaPushin R, et al. Pilot experience with continuous infusion alemtuzumab in patients with fludarabine-refractory chronic lymphocytic leukemia. Eur J Haematol. 2008;80:296–298. - PubMed
-
- Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute-Sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

