anti-Diastereo- and enantioselective carbonyl (hydroxymethyl)allylation from the alcohol or aldehyde oxidation level: allyl carbonates as allylmetal surrogates
- PMID: 20225853
- PMCID: PMC2848290
- DOI: 10.1021/ja100949e
anti-Diastereo- and enantioselective carbonyl (hydroxymethyl)allylation from the alcohol or aldehyde oxidation level: allyl carbonates as allylmetal surrogates
Abstract
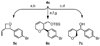
Enantioselective transfer hydrogenation of carbonate 1a in the presence of aromatic, allylic, or aliphatic alcohols 2a-2i employing a cyclometalated iridium C,O-benzoate derived from allyl acetate, 4-cyano-3-nitrobenzoic acid and (S)-SEGPHOS delivers products of (hydroxymethyl)allylation 4a-4i in good isolated yields (60-74%), good anti-diastereoselectivities (5:1-10:1 dr) and exceptional levels of enantiocontrol (93-99% ee). Under identical reaction conditions, but in the presence of isopropanol, aldehydes 3a-3i are converted to an equivalent set of adducts 4a-4i in good isolated yields (58-74%), good anti-diastereoselectivities (4:1-14:1 dr), and exceptional levels of enantiocontrol (95-99% ee). Thus, identical sets of adducts 4a-4i are produced with equal facility from the alcohol or aldehyde oxidation level. These studies represent the first general method for enantioselective carbonyl (hydroxymethyl)allylation, a process that has no highly stereoselective counterpart in conventional allylmetal chemistry.
Figures
Similar articles
-
Iridium-catalyzed anti-diastereo- and enantioselective carbonyl (trimethylsilyl)allylation from the alcohol or aldehyde oxidation level.J Am Chem Soc. 2010 Jul 7;132(26):9153-6. doi: 10.1021/ja103299f. J Am Chem Soc. 2010. PMID: 20540509 Free PMC article.
-
Enantioselective iridium-catalyzed carbonyl allylation from the alcohol or aldehyde oxidation level using allyl acetate as an allyl metal surrogate.J Am Chem Soc. 2008 May 21;130(20):6340-1. doi: 10.1021/ja802001b. Epub 2008 Apr 29. J Am Chem Soc. 2008. PMID: 18444616 Free PMC article.
-
Enantioselective iridium-catalyzed carbonyl allylation from the alcohol or aldehyde oxidation level via transfer hydrogenative coupling of allyl acetate: departure from chirally modified allyl metal reagents in carbonyl addition.J Am Chem Soc. 2008 Nov 5;130(44):14891-9. doi: 10.1021/ja805722e. Epub 2008 Oct 8. J Am Chem Soc. 2008. PMID: 18841896 Free PMC article.
-
Enantioselective iridium-catalyzed carbonyl allylation from the alcohol oxidation level via transfer hydrogenation: minimizing pre-activation for synthetic efficiency.Chem Commun (Camb). 2009 Dec 21;(47):7278-87. doi: 10.1039/b917243m. Epub 2009 Oct 16. Chem Commun (Camb). 2009. PMID: 20024203 Free PMC article. Review.
-
Asymmetric Iridium-Catalyzed C-C Coupling of Chiral Diols via Site-Selective Redox-Triggered Carbonyl Addition.Top Curr Chem. 2016;372:85-101. doi: 10.1007/128_2015_651. Top Curr Chem. 2016. PMID: 26187028 Free PMC article. Review.
Cited by
-
C-Propargylation Overrides O-Propargylation in Reactions of Propargyl Chloride with Primary Alcohols: Rhodium-Catalyzed Transfer Hydrogenation.Angew Chem Int Ed Engl. 2016 Aug 1;55(32):9207-11. doi: 10.1002/anie.201603575. Epub 2016 Jun 20. Angew Chem Int Ed Engl. 2016. PMID: 27321353 Free PMC article.
-
Catalytic Enantioselective C-C Coupling of Alcohols for Polyketide Total Synthesis beyond Chiral Auxiliaries and Premetalated Reagents.Chem Rev. 2024 Dec 25;124(24):13715-13735. doi: 10.1021/acs.chemrev.4c00858. Epub 2024 Dec 6. Chem Rev. 2024. PMID: 39642170 Review.
-
Nickel-catalyzed asymmetric reductive aryl-allylation of unactivated alkenes.Chem Sci. 2021 Apr 9;12(19):6712-6718. doi: 10.1039/d1sc01115d. Chem Sci. 2021. PMID: 34040746 Free PMC article.
-
Iridium-catalyzed anti-diastereo- and enantioselective carbonyl (trimethylsilyl)allylation from the alcohol or aldehyde oxidation level.J Am Chem Soc. 2010 Jul 7;132(26):9153-6. doi: 10.1021/ja103299f. J Am Chem Soc. 2010. PMID: 20540509 Free PMC article.
-
Catalytic enantioselective Grignard Nozaki-Hiyama methallylation from the alcohol oxidation level: chloride compensates for π-complex instability.Chem Commun (Camb). 2011 Sep 28;47(36):10028-30. doi: 10.1039/c1cc14392a. Epub 2011 Aug 10. Chem Commun (Camb). 2011. PMID: 21829853 Free PMC article.
References
-
-
Tedanolide, 13-deoxytedanolide and the myriaporones incorporate the hydroxymethyl 1,3-diol motif. For total syntheses, see: Pérez M, del Pozo C, Reyes F, Rodríguez A, Francesch A, Echavarren AM, Cuevas C. Angew. Chem., Int. Ed. 2004;43:1724. Taylor RE, Fleming KN. Angew. Chem., Int. Ed. 2004;43:1728. Julian LD, Newcom JS, Roush WR. J. Am. Chem. Soc. 2005;127:6186. Ehrlich G, Hassfeld J, Eggert U, Kalesse M. J. Am. Chem. Soc. 2006;128:14038. Smith AB, III, Lee D. J. Am. Chem. Soc. 2007;129:10957. Dunetz JR, Julian LD, Newcom JS, Roush WR. J. Am. Chem. Soc. 2008;130:16407. Ehrlich G, Hassfeld J, Eggert U, Kalesse M. Chem. Eur. J. 2008;14:2232.
-
-
-
Tylosin, its aglycone tylonolide, and O-mycinosyltylonolide incorporate the hydroxymethyl 1,3-diol motif. For total syntheses, see: Tatsuta K, Amemiya Y, Kanemura Y, Kinoshita M. Tetrahedron Lett. 1981;22:3997. Tatsuta K, Amemiya Y, Kanemura Y, Takahashi H, Kinoshita M. Tetrahedron Lett. 1982;23:3375. Masamune S, Lu LD-L, Jackson WP, Kaiho T, Toyoda T. J. Am. Chem. Soc. 1982;104:5523. Grieco PA, Inanaga J, Lin N-H, Yanami T. J. Am. Chem. Soc. 1982;104:5781. Nicolaou KC, Seitz SP, Pavia MR. J. Am. Chem. Soc. 1982;104:2030. Tanaka T, Oikawa Y, Hamada T, Yonemitsu O. Tetrahedron Lett. 1986;27:3651. Tanaka T, Oikawa Y, Hamada T, Yonemitsu O. Chem. Pharm. Bull. 1987;35:2219.
-
-
-
The mycinamicins incorporate the hydroxymethyl 1,3-diol motif. For total syntheses, see: Suzuki K, Matsumoto T, Tsuchihashi G-i. Chem. Lett. 1987:113. Matsumoto T, Maeta H, Suzuki K, Tsuchihashi G-i. Tetrahedron Lett. 1988;29:3575.
-
-
-
For other natural products that incorporate the hydroxymethyl 1,3-diol motif, see Supporting Information.
-
-
-
For (hydroxymethyl)allylation via palladium catalyzed reductive coupling of allylic carboxylates, see: Masuyama Y, Takahara JP, Kurusu Y. J. Am. Chem. Soc. 1988;110:4473. Masuyama Y, Otake K, Kurusu Y. Tetrahedron Lett. 1988;29:3563. Takahara JP, Masuyama Y, Kurusu Y. J. Am. Chem. Soc. 1992;114:2577.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

