Regulation of TFIIIB during F9 cell differentiation
- PMID: 20226026
- PMCID: PMC2842266
- DOI: 10.1186/1471-2199-11-21
Regulation of TFIIIB during F9 cell differentiation
Abstract
Background: Differentiation of F9 embryonal carcinoma (EC) cells into parietal endoderm (PE) provides a tractable model system for studying molecular events during early and inaccessible stages of murine development. PE formation is accompanied by extensive changes in gene expression both in vivo and in culture. One of the most dramatic is the ~10-fold decrease in transcriptional output by RNA polymerase (pol) III. This has been attributed to changes in activity of TFIIIB, a factor that is necessary and sufficient to recruit pol III to promoters. The goal of this study was to identify molecular changes that can account for the low activity of TFIIIB following F9 cell differentiation.
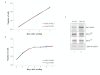
Results: Three essential subunits of TFIIIB decrease in abundance as F9 cells differentiate; these are Brf1 and Bdp1, which are pol III-specific, and TBP, which is also used by pols I and II. The decreased levels of Brf1 and Bdp1 proteins can be explained by reduced expression of the corresponding mRNAs. However, this is not the case for TBP, which is regulated post-transcriptionally. In proliferating cells, pol III transcription is stimulated by the proto-oncogene product c-Myc and the mitogen-activated protein kinase Erk, both of which bind to TFIIIB. However, c-Myc levels fall during differentiation and Erk becomes inactive through dephosphorylation. The diminished abundance of TFIIIB is therefore likely to be compounded by changes to these positive regulators that are required for its full activity. In addition, PE cells have elevated levels of the retinoblastoma protein RB, which is known to bind and repress TFIIIB.
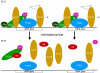
Conclusion: The low activity of TFIIIB in PE can be attributed to a combination of changes, any one of which could be sufficient to inhibit pol III transcription. Declining levels of essential TFIIIB subunits and of activators that are required for maximal TFIIIB activity are accompanied by an increase in a potent repressor of TFIIIB. These events provide fail-safe guarantees to ensure that pol III output is appropriate to the diminished metabolic requirements of terminally differentiated cells.
Figures



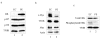
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

