Immunity of replicating Mu to self-integration: a novel mechanism employing MuB protein
- PMID: 20226074
- PMCID: PMC2837660
- DOI: 10.1186/1759-8753-1-8
Immunity of replicating Mu to self-integration: a novel mechanism employing MuB protein
Abstract
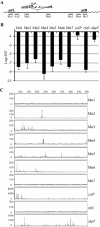
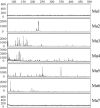
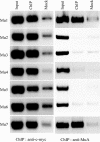
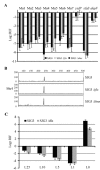
We describe a new immunity mechanism that protects actively replicating/transposing Mu from self-integration. We show that this mechanism is distinct from the established cis-immunity mechanism, which operates by removal of MuB protein from DNA adjacent to Mu ends. MuB normally promotes integration into DNA to which it is bound, hence its removal prevents use of this DNA as target. Contrary to what might be expected from a cis-immunity mechanism, strong binding of MuB was observed throughout the Mu genome. We also show that the cis-immunity mechanism is apparently functional outside Mu ends, but that the level of protection offered by this mechanism is insufficient to explain the protection seen inside Mu. Thus, both strong binding of MuB inside and poor immunity outside Mu testify to a mechanism of immunity distinct from cis-immunity, which we call 'Mu genome immunity'. MuB has the potential to coat the Mu genome and prevent auto-integration as previously observed in vitro on synthetic A/T-only DNA, where strong MuB binding occluded the entire bound region from Mu insertions. The existence of two rival immunity mechanisms within and outside the Mu genome, both employing MuB, suggests that the replicating Mu genome must be segregated into an independent chromosomal domain. We propose a model for how formation of a 'Mu domain' may be aided by specific Mu sequences and nucleoid-associated proteins, promoting polymerization of MuB on the genome to form a barrier against self-integration.
Figures


Similar articles
-
MuB gives a new twist to target DNA selection.Mob Genet Elements. 2013 Sep 1;3(5):e27515. doi: 10.4161/mge.27515. Epub 2013 Dec 12. Mob Genet Elements. 2013. PMID: 24478936 Free PMC article.
-
Transposable Phage Mu.Microbiol Spectr. 2014 Oct;2(5):10.1128/microbiolspec.MDNA3-0007-2014. doi: 10.1128/microbiolspec.MDNA3-0007-2014. Microbiol Spectr. 2014. PMID: 26104374 Free PMC article. Review.
-
Deep sequencing reveals new roles for MuB in transposition immunity and target-capture, and redefines the insular Ter region of E. coli.Mob DNA. 2020 Jul 9;11:26. doi: 10.1186/s13100-020-00217-9. eCollection 2020. Mob DNA. 2020. PMID: 32670425 Free PMC article.
-
Congruence of in vivo and in vitro insertion patterns in hot E. coli gene targets of transposable element Mu: opposing roles of MuB in target capture and integration.J Mol Biol. 2008 Jul 18;380(4):598-607. doi: 10.1016/j.jmb.2008.05.032. Epub 2008 May 20. J Mol Biol. 2008. PMID: 18556020 Free PMC article.
-
A call for replicating vector prime-protein boost strategies in HIV vaccine design.Expert Rev Vaccines. 2004 Aug;3(4 Suppl):S105-17. doi: 10.1586/14760584.3.4.s105. Expert Rev Vaccines. 2004. PMID: 15285710 Review.
Cited by
-
MuB gives a new twist to target DNA selection.Mob Genet Elements. 2013 Sep 1;3(5):e27515. doi: 10.4161/mge.27515. Epub 2013 Dec 12. Mob Genet Elements. 2013. PMID: 24478936 Free PMC article.
-
Transposable prophage Mu is organized as a stable chromosomal domain of E. coli.PLoS Genet. 2013 Nov;9(11):e1003902. doi: 10.1371/journal.pgen.1003902. Epub 2013 Nov 7. PLoS Genet. 2013. PMID: 24244182 Free PMC article.
-
Transposition Behavior Revealed by High-Resolution Description of Pseudomonas Aeruginosa Saltovirus Integration Sites.Viruses. 2018 May 7;10(5):245. doi: 10.3390/v10050245. Viruses. 2018. PMID: 29735891 Free PMC article.
-
MuA-mediated in vitro cloning of circular DNA: transpositional autointegration and the effect of MuB.Mol Genet Genomics. 2016 Jun;291(3):1181-91. doi: 10.1007/s00438-016-1175-2. Epub 2016 Feb 4. Mol Genet Genomics. 2016. PMID: 26847688
-
Transposable Phage Mu.Microbiol Spectr. 2014 Oct;2(5):10.1128/microbiolspec.MDNA3-0007-2014. doi: 10.1128/microbiolspec.MDNA3-0007-2014. Microbiol Spectr. 2014. PMID: 26104374 Free PMC article. Review.
References
-
- Chaconas G, Harshey RM. In: Mobile DNA II. Craig NL, Craigie R, Gellert M, Lambowitz AM, editor. Washington DC: ASM Press; 2002. Transposition of phage Mu DNA; pp. 384–402.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

