Transposable elements in a marginal plant population: temporal fluctuations provide new insights into genome evolution of wild diploid wheat
- PMID: 20226076
- PMCID: PMC2836003
- DOI: 10.1186/1759-8753-1-6
Transposable elements in a marginal plant population: temporal fluctuations provide new insights into genome evolution of wild diploid wheat
Abstract
Background: How new forms arise in nature has engaged evolutionary biologists since Darwin's seminal treatise on the origin of species. Transposable elements (TEs) may be among the most important internal sources for intraspecific variability. Thus, we aimed to explore the temporal dynamics of several TEs in individual genotypes from a small, marginal population of Aegilops speltoides. A diploid cross-pollinated grass species, it is a wild relative of the various wheat species known for their large genome sizes contributed by an extraordinary number of TEs, particularly long terminal repeat (LTR) retrotransposons. The population is characterized by high heteromorphy and possesses a wide spectrum of chromosomal abnormalities including supernumerary chromosomes, heterozygosity for translocations, and variability in the chromosomal position or number of 45S and 5S ribosomal DNA (rDNA) sites. We propose that variability on the morphological and chromosomal levels may be linked to variability at the molecular level and particularly in TE proliferation.
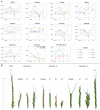
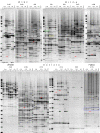
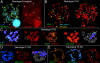
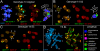
Results: Significant temporal fluctuation in the copy number of TEs was detected when processes that take place in small, marginal populations were simulated. It is known that under critical external conditions, outcrossing plants very often transit to self-pollination. Thus, three morphologically different genotypes with chromosomal aberrations were taken from a wild population of Ae. speltoides, and the dynamics of the TE complex traced through three rounds of selfing. It was discovered that: (i) various families of TEs vary tremendously in copy number between individuals from the same population and the selfed progenies; (ii) the fluctuations in copy number are TE-family specific; (iii) there is a great difference in TE copy number expansion or contraction between gametophytes and sporophytes; and (iv) a small percentage of TEs that increase in copy number can actually insert at novel locations and could serve as a bona fide mutagen.
Conclusions: We hypothesize that TE dynamics could promote or intensify morphological and karyotypical changes, some of which may be potentially important for the process of microevolution, and allow species with plastic genomes to survive as new forms or even species in times of rapid climatic change.
Figures


References
-
- Mayr E. Systematics and the origin of species. New York, USA: Columbia University Press; 1942.
-
- Grant V. Plant speciation. 2. New York, USA: Columbia University Press; 1981.
LinkOut - more resources
Full Text Sources

