Endocytosis of nanomedicines
- PMID: 20226220
- PMCID: PMC2902597
- DOI: 10.1016/j.jconrel.2010.01.036
Endocytosis of nanomedicines
Abstract
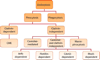
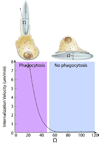
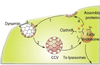
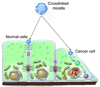
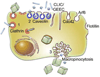
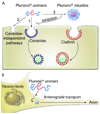
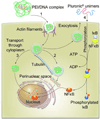
Novel nanomaterials are being developed to improve diagnosis and therapy of diseases through effective delivery of drugs, biopharmaceutical molecules and imaging agents to target cells in disease sites. Such diagnostic and therapeutic nanomaterials, also termed "nanomedicines", often require site-specific cellular entry to deliver their payload to sub-cellular locations hidden beneath cell membranes. Nanomedicines can employ multiple pathways for cellular entry, which are currently insufficiently understood. This review, first, classifies various mechanisms of endocytosis available to nanomedicines including phagocytosis and pinocytosis through clathrin-dependent and clathrin-independent pathways. Second, it describes the current experimental tools to study endocytosis of nanomedicines. Third, it provides specific examples from recent literature and our own work on endocytosis of nanomedicines. Finally, these examples are used to ascertain 1) the role of particle size, shape, material composition, surface chemistry and/or charge for utilization of a selected pathway(s); 2) the effect of cell type on the processing of nanomedicines; and 3) the effect of nanomaterial-cell interactions on the processes of endocytosis, the fate of the nanomedicines and the resulting cellular responses. This review will be useful to a diverse audience of students and scientists who are interested in understanding endocytosis of nanomedicines.
Copyright (c) 2010 Elsevier B.V. All rights reserved.
Figures



References
-
- Kabanov AV, Alakhov VY. Pluronic (R) block copolymers in drug delivery: From micellar nanocontainers to biological response modifiers. Critical Reviews in Therapeutic Drug Carrier Systems. 2002;19:1–72. - PubMed
-
- Duncan R. The dawning era of polymer therapeutics. Nature Review Drug Discovery. 2003;2:347–360. - PubMed
-
- Farokhzad OC, Langer R. Nanomedicine: Developing smarter therapeutic and diagnostic modalities. Advanced Drug Delivery Reviews. 2006;58:1456–1459. - PubMed
-
- Davis ME, Chen Z, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nature Review Drug Discovery. 2008;7:771–782. - PubMed
-
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nature Nanotechnology. 2007;2:751–760. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

