Role of glycosylation of Notch in development
- PMID: 20226260
- PMCID: PMC2898917
- DOI: 10.1016/j.semcdb.2010.03.003
Role of glycosylation of Notch in development
Abstract
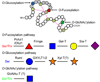
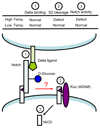
The Notch pathway is one of the major signaling pathways required for proper development in metazoans. Notch activity is regulated at numerous levels, and increasing evidence reveals the importance of "protein glycosylation" (modification of Notch receptors with sugars) for its regulation. In this review we summarize the significance of the Notch pathway in development and the players responsible for its glycosylation, and then discuss the molecular mechanisms by which protein glycosylation may regulate Notch function.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Significance of glycosylation in Notch signaling.Biochem Biophys Res Commun. 2014 Oct 17;453(2):235-42. doi: 10.1016/j.bbrc.2014.05.115. Epub 2014 Jun 6. Biochem Biophys Res Commun. 2014. PMID: 24909690 Free PMC article. Review.
-
Regulation of Notch signaling by glycosylation.Curr Opin Struct Biol. 2007 Oct;17(5):530-5. doi: 10.1016/j.sbi.2007.09.007. Epub 2007 Oct 25. Curr Opin Struct Biol. 2007. PMID: 17964136 Free PMC article. Review.
-
O-fucose monosaccharide of Drosophila Notch has a temperature-sensitive function and cooperates with O-glucose glycan in Notch transport and Notch signaling activation.J Biol Chem. 2015 Jan 2;290(1):505-19. doi: 10.1074/jbc.M114.616847. Epub 2014 Nov 5. J Biol Chem. 2015. PMID: 25378397 Free PMC article.
-
Regulation of Notch Function by O-Glycosylation.Adv Exp Med Biol. 2018;1066:59-78. doi: 10.1007/978-3-319-89512-3_4. Adv Exp Med Biol. 2018. PMID: 30030822 Review.
-
Notch signaling in normal and disease States: possible therapies related to glycosylation.Curr Mol Med. 2007 Jun;7(4):427-45. doi: 10.2174/156652407780831593. Curr Mol Med. 2007. PMID: 17584081
Cited by
-
The Abelson kinase and the Nedd4 family E3 ligases co-regulate Notch trafficking to limit signaling.J Cell Biol. 2025 Jun 2;224(6):e202407066. doi: 10.1083/jcb.202407066. Epub 2025 Apr 4. J Cell Biol. 2025. PMID: 40183942
-
PRMT1-catalyzed NUSAP1 methylation enhances Notch2 signaling and 5-FU resistance in gastric cancer.Cell Death Dis. 2025 May 20;16(1):404. doi: 10.1038/s41419-025-07723-9. Cell Death Dis. 2025. PMID: 40393971 Free PMC article.
-
Multiple members of the UDP-GalNAc: polypeptide N-acetylgalactosaminyltransferase family are essential for viability in Drosophila.J Biol Chem. 2012 Feb 17;287(8):5243-52. doi: 10.1074/jbc.M111.306159. Epub 2011 Dec 7. J Biol Chem. 2012. PMID: 22157008 Free PMC article.
-
Genetic characterization of an isolate of canine distemper virus from a Tibetan Mastiff in China.Virus Genes. 2014 Aug;49(1):45-57. doi: 10.1007/s11262-014-1062-z. Epub 2014 Apr 2. Virus Genes. 2014. PMID: 24691820 Free PMC article.
-
Golgi glycosylation.Cold Spring Harb Perspect Biol. 2011 Apr 1;3(4):a005199. doi: 10.1101/cshperspect.a005199. Cold Spring Harb Perspect Biol. 2011. PMID: 21441588 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

