Comparison of the immune responses induced by oral immunization of mice with Lactobacillus casei-expressing porcine parvovirus VP2 and VP2 fused to Escherichia coli heat-labile enterotoxin B subunit protein
- PMID: 20226529
- PMCID: PMC7112623
- DOI: 10.1016/j.cimid.2010.02.004
Comparison of the immune responses induced by oral immunization of mice with Lactobacillus casei-expressing porcine parvovirus VP2 and VP2 fused to Escherichia coli heat-labile enterotoxin B subunit protein
Abstract
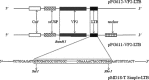
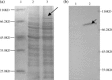
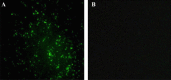
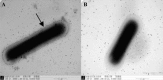
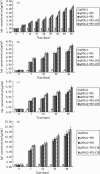
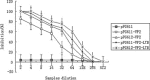
The major structural protein VP2 of porcine parvovirus (PPV) was used as the model parvovirus antigen, which has been expressed in Lactobacillus casei fusing with Escherichia coli heat-labile enterotoxin B subunit (LTB) as mucosal adjuvant. The VP2-LTB DNA fragment was cloned into vector pPG611 or pPG612 to generated inducible surface-displayed and secretion expression systems based on xylose promoter, designated as rLc:pPG611-VP2-LTB (recombinant L. casei) and rLc:pPG612-VP2-LTB, respectively. Expression of the fusion protein was verified by SDS-PAGE, Western blot immunofluorescence and electron microscopy. It was observed that the level of IgG or sIgA from mice orally immunized with VP2-LTB was higher than that from mice received VP2 and negative control, which demonstrated significantly statistically different. Especially, the titer of IgG or sIgA in mice immunized with rLc:pPG612-VP2-LTB is the highest in this study. In summary, LTB as mucosal adjuvant was able to effectively facilitate induction of mucosal and systemic immunity by L. casei-expressing VP2 fusion protein.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Recombinant porcine rotavirus VP4 and VP4-LTB expressed in Lactobacillus casei induced mucosal and systemic antibody responses in mice.BMC Microbiol. 2009 Dec 4;9:249. doi: 10.1186/1471-2180-9-249. BMC Microbiol. 2009. PMID: 19958557 Free PMC article.
-
[Co-expression of CSFV T cell epitope E290 peptide and PPV VP2 protein in Lactobacillus casei and determination of specific antibodies in immunized mice].Wei Sheng Wu Xue Bao. 2007 Aug;47(4):667-72. Wei Sheng Wu Xue Bao. 2007. PMID: 17944369 Chinese.
-
Construction of recombinant Lactobacillus casei efficiently surface displayed and secreted porcine parvovirus VP2 protein and comparison of the immune responses induced by oral immunization.Immunology. 2008 May;124(1):68-75. doi: 10.1111/j.1365-2567.2007.02738.x. Epub 2007 Nov 22. Immunology. 2008. PMID: 18034821 Free PMC article.
-
[Escherichia coli heat-labile enterotoxin B subunit enhances the immune response against canine parvovirus VP2 in mice immunized by VP2 DNA vaccine].Wei Sheng Wu Xue Bao. 2011 Jan;51(1):91-7. Wei Sheng Wu Xue Bao. 2011. PMID: 21465794 Chinese.
-
Oral Immunization with Lactobacillus casei Expressing the Porcine Circovirus Type 2 Cap and LTB Induces Mucosal and Systemic Antibody Responses in Mice.Viruses. 2021 Jul 5;13(7):1302. doi: 10.3390/v13071302. Viruses. 2021. PMID: 34372508 Free PMC article.
Cited by
-
Incorporation of membrane-anchored flagellin or Escherichia coli heat-labile enterotoxin B subunit enhances the immunogenicity of rabies virus-like particles in mice and dogs.Front Microbiol. 2015 Mar 3;6:169. doi: 10.3389/fmicb.2015.00169. eCollection 2015. Front Microbiol. 2015. PMID: 25784906 Free PMC article.
-
Chromosomal insertions in the Lactobacillus casei upp gene that are useful for vaccine expression.Appl Environ Microbiol. 2014 Jun;80(11):3321-6. doi: 10.1128/AEM.00175-14. Epub 2014 Mar 21. Appl Environ Microbiol. 2014. PMID: 24657853 Free PMC article.
-
Recovery of recombinant Mycobacterium tuberculosis antigens fused with cell wall-anchoring motif (LysM) from inclusion bodies using non-denaturing reagent (N-laurylsarcosine).BMC Biotechnol. 2019 May 14;19(1):27. doi: 10.1186/s12896-019-0522-x. BMC Biotechnol. 2019. PMID: 31088425 Free PMC article.
-
Expression of bioactive porcine interferon-alpha in Lactobacillus casei.World J Microbiol Biotechnol. 2014 Sep;30(9):2379-86. doi: 10.1007/s11274-014-1663-7. Epub 2014 May 13. World J Microbiol Biotechnol. 2014. PMID: 24818858
-
Immune responses induced by recombinant Lactobacillus plantarum expressing the spike protein derived from transmissible gastroenteritis virus in piglets.Appl Microbiol Biotechnol. 2018 Oct;102(19):8403-8417. doi: 10.1007/s00253-018-9205-0. Epub 2018 Jul 18. Appl Microbiol Biotechnol. 2018. PMID: 30022263 Free PMC article.
References
-
- Wilhelm S., Zimmermann P., Selbitz H.J., Truyen U. Real-time PCR protocol for the detection of porcine parvovirus in field samples. J Virol Methods. 2006;134:257–260. - PubMed
-
- Bergeron J., Menezes J., Tijssen J. Genomic organization and mapping of transcription and translation products of the NADL-2 strain of porcine parvovirus. Virology. 1993;197:86–98. - PubMed
-
- Allan G.M., Kennedy S., McNeilly F., Foster J.C., Ellis J.A., Krakowka S.J. Experimental reproduction of severe wasting disease by co-infection of pigs with porcine circovirus and porcine parvovirus. J Comp Pathol. 1999;121(1):1–11. - PubMed
-
- Ellis J.A., Bratanich A., Clark E.G., Allan G., Meehan B., Haines D.M. Coinfection by porcine circoviruses and porcine parvovirus in pigs with naturally acquired postweaning multisystemic wasting syndrome. J Vet Diagn Invest. 2000;12(1):21–27. - PubMed
-
- Parke C.R., Burgess G.W. An economic assessment of porcine parvovirus vaccination. Vet J. 1993;70(5):177–180. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

