Ten years of NAD-dependent SIR2 family deacetylases: implications for metabolic diseases
- PMID: 20226541
- PMCID: PMC3526941
- DOI: 10.1016/j.tips.2010.02.003
Ten years of NAD-dependent SIR2 family deacetylases: implications for metabolic diseases
Abstract
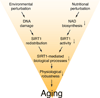
Since the discovery of NAD-dependent deacetylase activity of the silent information regulator-2 (SIR2) family ('sirtuins'), many exciting connections between protein deacetylation and energy metabolism have been revealed. The importance of sirtuins in the regulation of many fundamental biological responses to various nutritional and environmental stimuli has been firmly established. Sirtuins have also emerged as critical regulators for aging and longevity in model organisms. Their absolute requirement of NAD has revived an enthusiasm in the study of mammalian biosynthesis of NAD. Sirtuin-targeted pharmaceutical and nutriceutical interventions against age-associated diseases are also on the horizon. This review summarizes the recent progress in sirtuin research (particularly in mammalian sirtuin biology) and re-evaluates the connection between sirtuins, metabolism, and age-associated diseases (e.g., type-2 diabetes) to set a basis for the next ten years of sirtuin research.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures


References
-
- Tsang AW, Escalante-Semerena JC. CobB, a new member of the SIR2 family of eucaryotic regulatory proteins, is required to compensate for the lack of nicotinate mononucleotide: 5,6-dimethylbenzimidazole phosphoribosyltransferase activity in cobT mutants during cobalamin biosynthesis in Salmonella typhimurium LT2. J. Biol. Chem. 1998;273:31788–31794. - PubMed
-
- Trzebiatowski JR, Escalante-Semerena JC. Purification and characterization of CobT, the nicotinate-mononucleotide:5,6-dimethylbenzimidazole phosphoribosyltransferase enzyme from Salmonella typhimurium LT2. J. Biol. Chem. 1997;272:17662–17667. - PubMed
-
- Brachmann CB, et al. The SIR2 gene family, conserved from bacteria to humans, functions in silencing, cell cycle progression, and chromosome stability. Genes Dev. 1995;9:2888–2902. - PubMed
-
- Frye RA. Characterization of five human cDNAs with homology to yeast SIR2 gene: Sir2-like proteins (Sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem. Biophys. Res. Commun. 1999;260:273–279. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

