PRL-2 increases Epo and IL-3 responses in hematopoietic cells
- PMID: 20226699
- PMCID: PMC2847026
- DOI: 10.1016/j.bcmd.2010.02.013
PRL-2 increases Epo and IL-3 responses in hematopoietic cells
Abstract
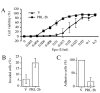
Dual specificity protein tyrosine phosphatase PRL-2 is overexpressed in pediatric acute myeloid leukemia (AML) and is located at human chromosome 1p35, a region often rearranged or amplified in malignant lymphoma and B-cell chronic lymphocytic leukemia (B-CLL). Little is known of the significance of PRL-2 expression in hematopoietic malignancies. Herein we demonstrated that ectopic expression of PRL-2 in murine pre-B-cell line Baf3ER and mouse bone marrow cells induced key features associated with malignant progression and metastasis. PRL-2-transfected Baf3ER cells had augmented growth responses to hematopoietic growth factors Epo or IL-3 with shortened cell cycle, reduced requirement (5x) for Epo in cell survival, increased cell migration (3x), reduced cell adhesion (5x), and conversion to an immature cell morphology in association with increased expression (3x) of stem cell marker Bmi-1. When transduced into mouse bone marrow cells, PRL-2 increased Epo-induced colony formation (4x) and gave rise to larger colonies. These observations provide evidences implicating PRL-2 as a pathogenic molecule in hematopoietic malignancies and suggest its potential as a novel therapeutic target.
Conflict of interest statement
Figures





Similar articles
-
Hematopoietic cell phosphatase negatively regulates erythropoietin-induced hemoglobinization in erythroleukemic SKT6 cells.Blood. 1997 Sep 15;90(6):2175-87. Blood. 1997. PMID: 9310468
-
The critical role of SRC homology domain 2-containing tyrosine phosphatase-1 in recombinant human erythropoietin hyporesponsive anemia in chronic hemodialysis patients.J Am Soc Nephrol. 2004 Dec;15(12):3215-24. doi: 10.1097/01.ASN.0000145457.73744.24. J Am Soc Nephrol. 2004. PMID: 15579525
-
SHP-1 phosphatase C-terminus interacts with novel substrates p32/p30 during erythropoietin and interleukin-3 mitogenic responses.Blood. 1998 May 15;91(10):3746-55. Blood. 1998. PMID: 9573011
-
Apoptosis and hematopoiesis in murine fetal liver.Blood. 1993 Jan 15;81(2):373-84. Blood. 1993. PMID: 7678514
-
Expression of FLT3 receptor and response to FLT3 ligand by leukemic cells.Leukemia. 1996 Apr;10(4):588-99. Leukemia. 1996. PMID: 8618433 Review.
Cited by
-
Expression of phosphatase of regenerating liver-3 is associated with prognosis of Wilms' tumor.Onco Targets Ther. 2017 Jan 10;10:311-317. doi: 10.2147/OTT.S107076. eCollection 2017. Onco Targets Ther. 2017. PMID: 28138254 Free PMC article.
-
PRL2/PTP4A2 phosphatase is important for hematopoietic stem cell self-renewal.Stem Cells. 2014 Jul;32(7):1956-67. doi: 10.1002/stem.1672. Stem Cells. 2014. PMID: 24753135 Free PMC article.
-
Phosphatase of regenerating liver in hematopoietic stem cells and hematological malignancies.Cell Cycle. 2014;13(18):2827-35. doi: 10.4161/15384101.2014.954448. Cell Cycle. 2014. PMID: 25486470 Free PMC article. Review.
-
Molecular characterization of adipose tissue in the African elephant (Loxodonta africana).PLoS One. 2014 Mar 14;9(3):e91717. doi: 10.1371/journal.pone.0091717. eCollection 2014. PLoS One. 2014. PMID: 24633017 Free PMC article.
References
-
- Zeng Q, Hong W, Tan YH. Mouse PRL-2 and PRL-3, two potentially prenylated protein tyrosine phosphatases homologous to PRL-1. Biochem Biophys Res Commun. 1998;244:421–427. - PubMed
-
- Werner SR, Lee PA, DeCamp MW, Crowell DN, Randall SK, Crowell PL. Enhanced cell cycle progression and down regulation of p21(Cip1/Waf1) by PRL tyrosine phosphatases. Cancer Lett. 2003;202:201–211. - PubMed
-
- Cates CA, Michael RL, Stayrook KR, Harvey KA, Burke YD, Randall SK, Crowell PL, Crowell DN. Prenylation of oncogenic human PTP(CAAX) protein tyrosine phosphatases. Cancer Lett. 1996;110:49–55. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

