Respiratory compliance in preterm infants after a single rescue course of antenatal steroids: a randomized controlled trial
- PMID: 20227053
- PMCID: PMC2878893
- DOI: 10.1016/j.ajog.2010.01.038
Respiratory compliance in preterm infants after a single rescue course of antenatal steroids: a randomized controlled trial
Abstract
Objective: To compare respiratory compliance and functional residual capacity in infants randomized to a rescue course of antenatal steroids vs placebo.
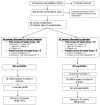
Study design: Randomized, double-blinded trial. Pregnant women > or =14 days after initial antenatal steroids were randomized to rescue antenatal steroids or placebo. The primary outcomes were measurements of respiratory compliance and functional residual capacity. This study is registered with clinicaltrials.gov (NCT00669383).
Results: Forty-four mothers (56 infants) received rescue antenatal steroids and 41 mothers (57 infants) received placebo. There was no significant difference in birthweight, or head circumference. Infants in the rescue group had an increased respiratory compliance (1.21 vs 1.01 mL/cm H(2)O/kg; adjusted 95% confidence interval, 0.01-0.49; P = .0433) compared with placebo. 13% in the rescue vs 29% in the placebo group required > or =30% oxygen (P < .05). Patients delivered at < or =34 weeks had greater pulmonary benefits.
Conclusion: Infants randomized to rescue antenatal steroids have a significantly increased respiratory compliance compared with placebo.
Copyright 2010 Mosby, Inc. All rights reserved.
Conflict of interest statement
Figures
References
-
- Jobe AH, Bancalari E. NICHD/NHLBI/ORD Workshop Summary. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. - PubMed
-
- National Institutes of Health Consensus Development Conference Statement. Effect of corticosteroids for fetal maturation on perinatal outcomes, February 28-March 2, 1994. Am J Obstet Gynecol. 1995;173:246–252.
-
- Crowley PA. Antenatal corticosteroid therapy: A meta-analysis of the randomized trials, 1972 to 1994. Am J Obstet Gynecol. 1995;173:322–334. - PubMed
-
- National Institutes of Health Consensus Development Panel. Antenatal corticosteroids revisited: Repeat courses-NIH Consensus Development Conference Statement, August 17-18, 2000. Obstet Gynecol. 2001;98:144–150. - PubMed
-
- Crowther CA, Haslam RR, Hiller JE, Doyle LW, Robinson JS Australasian Collaborative Trial of Repeat Doses of Steroids (ACTORDS) Study Group. Neonatal respiratory distress syndrome after repeat exposure to antenatal corticosteroids: A randomised controlled trial. Lancet. 2006;367:1913–1919. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical