Topological layers in the HIV-1 gp120 inner domain regulate gp41 interaction and CD4-triggered conformational transitions
- PMID: 20227370
- PMCID: PMC2854584
- DOI: 10.1016/j.molcel.2010.02.012
Topological layers in the HIV-1 gp120 inner domain regulate gp41 interaction and CD4-triggered conformational transitions
Abstract
The entry of human immunodeficiency virus (HIV-1) into cells is initiated by binding of the gp120 exterior envelope glycoprotein to the receptor, CD4. How does CD4 binding trigger conformational changes in gp120 that allow the gp41 transmembrane envelope glycoprotein to mediate viral-cell membrane fusion? The transition from the unliganded to the CD4-bound state is regulated by two potentially flexible topological layers (layers 1 and 2) in the gp120 inner domain. Both layers apparently contribute to the noncovalent association of unliganded gp120 with gp41. After CD4 makes initial contact with the gp120 outer domain, layer 1-layer 2 interactions strengthen gp120-CD4 binding by reducing the off rate. Layer 1-layer 2 interactions also destabilize the activated state induced on HIV-1 by treatment with soluble CD4. Thus, despite lack of contact with CD4, the gp120 inner-domain layers govern CD4 triggering by participating in conformational transitions within gp120 and regulating the interaction with gp41.
(c) 2010 Elsevier Inc. All rights reserved.
Figures

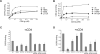


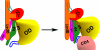
References
-
- Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. - PubMed
-
- Allan JS, Coligan JE, Barin F, McLane MF, Sodroski JG, Rosen CA, Haseltine WA, Lee TH, Essex M. Major glycoprotein antigens that induce antibodies in AIDS patients are encoded by HTLV-III. Science. 1985;228:1091–1094. - PubMed
-
- Allan JS, Strauss J, Buck DW. Enhancement of SIV infection with soluble receptor molecules. Science. 1990;247:1084–8. - PubMed
-
- Binley JM, Sanders RW, Clas B, Schuelke N, Master A, Guo Y, Kajumo F, Anselma DJ, Maddon PJ, Olson WC, et al. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J. Virol. 2000;74:627–43. - PMC - PubMed
-
- Chan DC, Fass D, Berger JM, Kim PS. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

