Folding anomalies of neuroligin3 caused by a mutation in the alpha/beta-hydrolase fold domain
- PMID: 20227402
- PMCID: PMC5582339
- DOI: 10.1016/j.cbi.2010.03.012
Folding anomalies of neuroligin3 caused by a mutation in the alpha/beta-hydrolase fold domain
Abstract
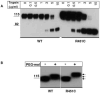
Proteins of the alpha/beta-hydrolase fold family share a common structural fold, but perform a diverse set of functions. We have been studying natural mutations occurring in association with congenital disorders in the alpha/beta-hydrolase fold domain of neuroligin (NLGN), butyrylcholinesterase (BChE), acetylcholinesterase (AChE). Starting from the autism-related R451C mutation in the alpha/beta-hydrolase fold domain of NLGN3, we had previously shown that the Arg to Cys substitution is responsible for endoplasmic reticulum (ER) retention of the mutant protein and that a similar trafficking defect is observed when the mutation is inserted at the homologous positions in AChE and BChE. Herein we show further characterization of the R451C mutation in NLGN3 when expressed in HEK-293, and by protease digestion sensitivity, we reveal that the phenotype results from protein misfolding. However, the presence of an extra Cys does not interfere with the formation of disulfide bonds as shown by reaction with PEG-maleimide and estimation of the molecular mass changes. These findings highlight the role of proper protein folding in protein processing and localization.
Copyright (c) 2010 Elsevier Ireland Ltd. All rights reserved.
Figures

Similar articles
-
Autism-associated R451C mutation in neuroligin3 leads to activation of the unfolded protein response in a PC12 Tet-On inducible system.Biochem J. 2016 Feb 15;473(4):423-34. doi: 10.1042/BJ20150274. Epub 2015 Nov 30. Biochem J. 2016. PMID: 26621873 Free PMC article.
-
Neuroligin trafficking deficiencies arising from mutations in the alpha/beta-hydrolase fold protein family.J Biol Chem. 2010 Sep 10;285(37):28674-82. doi: 10.1074/jbc.M110.139519. Epub 2010 Jul 8. J Biol Chem. 2010. PMID: 20615874 Free PMC article.
-
A mutation linked with autism reveals a common mechanism of endoplasmic reticulum retention for the alpha,beta-hydrolase fold protein family.J Biol Chem. 2006 Apr 7;281(14):9667-76. doi: 10.1074/jbc.M510262200. Epub 2006 Jan 24. J Biol Chem. 2006. PMID: 16434405
-
Processing of cholinesterase-like α/β-hydrolase fold proteins: alterations associated with congenital disorders.Protein Pept Lett. 2012 Feb;19(2):173-9. doi: 10.2174/092986612799080103. Protein Pept Lett. 2012. PMID: 21933121 Free PMC article. Review.
-
Alpha/beta hydrolase fold: an update.Protein Pept Lett. 2009;16(10):1137-48. doi: 10.2174/092986609789071298. Protein Pept Lett. 2009. PMID: 19508187 Review.
Cited by
-
Autism-associated R451C mutation in neuroligin3 leads to activation of the unfolded protein response in a PC12 Tet-On inducible system.Biochem J. 2016 Feb 15;473(4):423-34. doi: 10.1042/BJ20150274. Epub 2015 Nov 30. Biochem J. 2016. PMID: 26621873 Free PMC article.
-
Analyses of the autism-associated neuroligin-3 R451C mutation in human neurons reveal a gain-of-function synaptic mechanism.Mol Psychiatry. 2024 Jun;29(6):1620-1635. doi: 10.1038/s41380-022-01834-x. Epub 2022 Oct 24. Mol Psychiatry. 2024. PMID: 36280753 Free PMC article.
-
Exploring the role of BCHE in the onset of Diabetes, Obesity and Neurological Disorders.Bioinformation. 2012;8(6):276-80. doi: 10.6026/97320630008276. Epub 2012 Mar 31. Bioinformation. 2012. PMID: 22493536 Free PMC article.
-
A sex-specific association of common variants of neuroligin genes (NLGN3 and NLGN4X) with autism spectrum disorders in a Chinese Han cohort.Behav Brain Funct. 2011 May 14;7:13. doi: 10.1186/1744-9081-7-13. Behav Brain Funct. 2011. PMID: 21569590 Free PMC article.
-
Autism-associated neuroligin-3 mutations commonly disrupt tonic endocannabinoid signaling.Neuron. 2013 May 8;78(3):498-509. doi: 10.1016/j.neuron.2013.02.036. Epub 2013 Apr 11. Neuron. 2013. PMID: 23583622 Free PMC article.
References
-
- Ollis DL, Cheah E, Cygler M, Dijkstra B, Frolow F, Franken SM, Harel M, Remington SJ, Silman I, Schrag J, et al. The alpha/beta hydrolase fold. Protein Eng. 1992;5(3):197–211. - PubMed
-
- Carr PD, Ollis DL. Alpha/beta hydrolase fold: an update. Protein Pept Lett. 2009;16(10):1137–1148. - PubMed
-
- Ichtchenko K, Nguyen T, Sudhof TC. Structures, alternative splicing, and neurexin binding of multiple neuroligins. J Biol Chem. 1996;271(5):2676–2682. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

