The nuclear vitamin D receptor controls the expression of genes encoding factors which feed the "Fountain of Youth" to mediate healthful aging
- PMID: 20227497
- PMCID: PMC2906618
- DOI: 10.1016/j.jsbmb.2010.03.019
The nuclear vitamin D receptor controls the expression of genes encoding factors which feed the "Fountain of Youth" to mediate healthful aging
Abstract
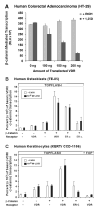
The nuclear vitamin D receptor (VDR) binds 1,25-dihydroxyvitamin D3 (1,25D), its high affinity renal endocrine ligand, to signal intestinal calcium and phosphate absorption plus bone remodeling, generating a mineralized skeleton free of rickets/osteomalacia with a reduced risk of osteoporotic fractures. 1,25D/VDR signaling regulates the expression of TRPV6, BGP, SPP1, LRP5, RANKL and OPG, while achieving feedback control of mineral ions to prevent age-related ectopic calcification by governing CYP24A1, PTH, FGF23, PHEX, and klotho transcription. Vitamin D also elicits numerous intracrine actions when circulating 25-hydroxyvitamin D3, the metabolite reflecting vitamin D status, is converted to 1,25D locally by extrarenal CYP27B1, and binds VDR to promote immunoregulation, antimicrobial defense, xenobiotic detoxification, anti-inflammatory/anticancer actions and cardiovascular benefits. VDR also affects Wnt signaling through direct interaction with beta-catenin, ligand-dependently blunting beta-catenin mediated transcription in colon cancer cells to attenuate growth, while potentiating beta-catenin signaling via VDR ligand-independent mechanisms in osteoblasts and keratinocytes to function osteogenically and as a pro-hair cycling receptor, respectively. Finally, VDR also drives the mammalian hair cycle in conjunction with the hairless corepressor by repressing SOSTDC1, S100A8/S100A9, and PTHrP. Hair provides a shield against UV-induced skin damage and cancer in terrestrial mammals, illuminating another function of VDR that facilitates healthful aging.
Copyright (c) 2010 Elsevier Ltd. All rights reserved.
Figures


References
-
- Whitfield GK, Jurutka PW, Haussler CA, Hsieh JC, Barthel TK, Jacobs ET, Encinas Dominguez C, Thatcher ML, Haussler MR. Nuclear vitamin D receptor: structure-function, molecular control of gene transcription, and novel bioactions. In: Feldman D, Pike JW, Glorieux FH, editors. Vitamin D. Elsevier Academic Press; Oxford, UK: 2005. pp. 219–261.
-
- Whitfield GK, Dang HTL, Schluter SF, Bernstein RM, Bunag T, Manzon LA, Hsieh G, Dominguez CE, Youson JH, Haussler MR, Marchalonis JJ. Cloning of a functional vitamin D receptor from the lamprey (Petromyzon marinus), an ancient vertebrate lacking a calcified skeleton and teeth. Endocrinology. 2003;144(6):2704–2716. - PubMed
-
- Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, Haussler MR, Mangelsdorf DJ. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296(5571):1313–1316. - PubMed
-
- Echchgadda I, Song CS, Roy AK, Chatterjee B. Dehydroepiandrosterone sulfotransferase is a target for transcriptional induction by the vitamin D receptor. Mol Pharmacol. 2004;65(3):720–729. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

