Repression of cellular retinoic acid-binding protein II during adipocyte differentiation
- PMID: 20228061
- PMCID: PMC2865280
- DOI: 10.1074/jbc.M110.110635
Repression of cellular retinoic acid-binding protein II during adipocyte differentiation
Abstract
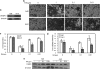
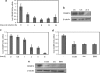
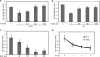
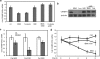
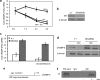
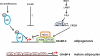
In preadipocytes, retinoic acid (RA) regulates gene expression by activating the nuclear RA receptor (RAR) and its cognate intracellular lipid-binding protein CRABP-II. It was previously reported that RA inhibits adipocyte differentiation but only when administered early during the differentiation program. The data presented here indicate that the diminished ability of RA to activate RAR following induction of differentiation stems from down-regulation of CRABP-II. The observations show that expression of CRABP-II in preadipocytes is repressed by all three components of the classical hormonal mixture that induces adipocyte differentiation, i.e. isobutylmethylxanthine, insulin, and dexamethasone. Isobutylmethylxanthine-dependent activation of protein kinase A triggered the phosphorylation of the transcription factor cAMP-response element-binding protein, which induced the expression of the cAMP-response element-binding protein family repressor cAMP-response element modulator. In turn, cAMP-response element modulator was found to associate with a cognate response element in the CRABP-II promoter and to repress CRABP-II expression. The data further show that CRABP-II is a direct target gene for the glucocorticoid receptor and that it is subjected to dexamethasone-induced glucocorticoid receptor-mediated repression during adipogenesis. Finally, the observations demonstrate that permanent repression of CRABP-II in mature adipocytes is exerted by the master regulator of adipocyte differentiation CCAAT/enhancer-binding protein alpha and is directly mediated through CCAAT/enhancer-binding protein alpha-response elements in the CRABP-II promoter. Taken together, the observations emphasize the important role of CRABP-II in regulating the transcriptional activity of RA through RAR, and they demonstrate that repression of this gene is critical for allowing adipogenesis to proceed.
Figures


References
-
- Green H., Meuth M. (1974) Cell 3, 127–133 - PubMed
-
- Green H., Kehinde O. (1975) Cell 5, 19–27 - PubMed
-
- Rosen E. D., Spiegelman B. M. (2000) Annu. Rev. Cell Dev. Biol. 16, 145–171 - PubMed
-
- Rosen E. D., Walkey C. J., Puigserver P., Spiegelman B. M. (2000) Genes Dev. 14, 1293–1307 - PubMed
-
- Zhang J. W., Klemm D. J., Vinson C., Lane M. D. (2004) J. Biol. Chem. 279, 4471–4478 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

