Myeloperoxidase-dependent inactivation of surfactant protein D in vitro and in vivo
- PMID: 20228064
- PMCID: PMC2878025
- DOI: 10.1074/jbc.M109.097048
Myeloperoxidase-dependent inactivation of surfactant protein D in vitro and in vivo
Abstract
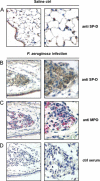
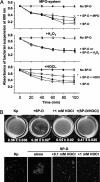
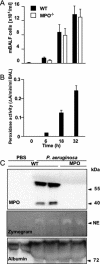
Surfactant protein D (SP-D) plays diverse and important roles in innate immunity and pulmonary homeostasis. Neutrophils and myeloperoxidase (MPO) colocalized with SP-D in a murine bacterial pneumonia model of acute inflammation, suggesting that MPO-derived reactive species might alter the function of SP-D. Exposure of SP-D to the complete MPO-H(2)O(2)-halide system caused loss of SP-D-dependent aggregating activity. Hypochlorous acid (HOCl), the major oxidant generated by MPO, caused a similar loss of aggregating activity, which was accompanied by the generation of abnormal disulfide-cross-linked oligomers. A full-length SP-D mutant lacking N-terminal cysteine residues and truncation mutants lacking the N-terminal domains were resistant to the oxidant-induced alterations in disulfide bonding. Mass spectroscopy of HOCl-treated human SP-D demonstrated several modifications, but none involved key ligand binding residues. There was detectable oxidation of cysteine 15, but no HOCl-induced cysteine modifications were observed in the C-terminal lectin domain. Together, the findings localize abnormal disulfide cross-links to the N-terminal domain. MPO-deficient mice showed decreased cross-linking of SP-D and increased SP-D-dependent aggregating activity in the pneumonia model. Thus, MPO-derived oxidants can lead to modifications of SP-D structure with associated alterations in its characteristic aggregating activity.
Figures







Similar articles
-
Modification of surfactant protein D by reactive oxygen-nitrogen intermediates is accompanied by loss of aggregating activity, in vitro and in vivo.FASEB J. 2009 May;23(5):1415-30. doi: 10.1096/fj.08-120568. Epub 2009 Jan 6. FASEB J. 2009. PMID: 19126597 Free PMC article.
-
Activity of pulmonary surfactant protein-D (SP-D) in vivo is dependent on oligomeric structure.J Biol Chem. 2001 Jun 1;276(22):19214-9. doi: 10.1074/jbc.M010191200. Epub 2001 Feb 9. J Biol Chem. 2001. PMID: 11278637
-
Biosynthesis of surfactant protein D. Contributions of conserved NH2-terminal cysteine residues and collagen helix formation to assembly and secretion.J Biol Chem. 1996 Aug 2;271(31):18912-9. doi: 10.1074/jbc.271.31.18912. J Biol Chem. 1996. PMID: 8756121
-
Surfactant proteins SP-A and SP-D in human health and disease.Arch Immunol Ther Exp (Warsz). 2005 Sep-Oct;53(5):399-417. Arch Immunol Ther Exp (Warsz). 2005. PMID: 16314824 Review.
-
Surfactant protein A and surfactant protein D variation in pulmonary disease.Immunobiology. 2007;212(4-5):381-416. doi: 10.1016/j.imbio.2007.01.003. Epub 2007 Feb 23. Immunobiology. 2007. PMID: 17544823 Review.
Cited by
-
Toll-like receptors and COVID-19: a two-faced story with an exciting ending.Future Sci OA. 2020 Jul 30;6(8):FSO605. doi: 10.2144/fsoa-2020-0091. Future Sci OA. 2020. PMID: 32974046 Free PMC article. No abstract available.
-
Surfactant Protein D in Respiratory and Non-Respiratory Diseases.Front Med (Lausanne). 2018 Feb 8;5:18. doi: 10.3389/fmed.2018.00018. eCollection 2018. Front Med (Lausanne). 2018. PMID: 29473039 Free PMC article. Review.
-
Revisiting surfactant protein D: an immune surveillance molecule bridging innate and adaptive immunity.Front Immunol. 2024 Dec 17;15:1491175. doi: 10.3389/fimmu.2024.1491175. eCollection 2024. Front Immunol. 2024. PMID: 39742280 Free PMC article. Review.
-
Oxidative damage of SP-D abolishes control of eosinophil extracellular DNA trap formation.J Leukoc Biol. 2018 Jul;104(1):205-214. doi: 10.1002/JLB.3AB1117-455R. Epub 2018 May 7. J Leukoc Biol. 2018. PMID: 29733456 Free PMC article.
-
Postexposure administration of a {beta}2-agonist decreases chlorine-induced airway hyperreactivity in mice.Am J Respir Cell Mol Biol. 2011 Jul;45(1):88-94. doi: 10.1165/rcmb.2010-0226OC. Epub 2010 Sep 20. Am J Respir Cell Mol Biol. 2011. PMID: 20855648 Free PMC article.
References
-
- Wright J. R. (2005) Nat. Rev. Immunol. 5, 58–68 - PubMed
-
- Hawgood S., Poulain F. R. (2001) Annu. Rev. Physiol. 63, 495–519 - PubMed
-
- LeVine A. M., Whitsett J. A. (2001) Microbes Infect. 3, 161–166 - PubMed
-
- Madsen J., Kliem A., Tornoe I., Skjodt K., Koch C., Holmskov U. (2000) J. Immunol. 164, 5866–5870 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1U01ES015676/ES/NIEHS NIH HHS/United States
- P01 HL029594/HL/NHLBI NIH HHS/United States
- HL-44015/HL/NHLBI NIH HHS/United States
- R01 HL044015/HL/NHLBI NIH HHS/United States
- DK17047/DK/NIDDK NIH HHS/United States
- R01 HL086798/HL/NHLBI NIH HHS/United States
- K99 HL091055/HL/NHLBI NIH HHS/United States
- K99HL-091055/HL/NHLBI NIH HHS/United States
- HL-086798/HL/NHLBI NIH HHS/United States
- R00 HL091055/HL/NHLBI NIH HHS/United States
- U01 ES015676/ES/NIEHS NIH HHS/United States
- P30 DK017047/DK/NIDDK NIH HHS/United States
- HL-29594/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

