Characterization of the transport, metabolism, and pharmacokinetics of the dopamine D3 receptor-selective fluorenyl- and 2-pyridylphenyl amides developed for treatment of psychostimulant abuse
- PMID: 20228156
- PMCID: PMC2879935
- DOI: 10.1124/jpet.109.165084
Characterization of the transport, metabolism, and pharmacokinetics of the dopamine D3 receptor-selective fluorenyl- and 2-pyridylphenyl amides developed for treatment of psychostimulant abuse
Abstract
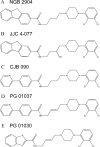
The recent discovery of novel high-affinity and selective dopamine D3 receptor (DA D3R) antagonists and partial agonists has provided tools with which to further elucidate the role DA D3R plays in substance abuse. The present study was conducted to evaluate the transport, metabolism, pharmacokinetics, and brain uptake of the DA D3R-selective fluorenyl amides, NGB 2904 [N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-9H-fluorene-2-carboxamide] fumarate) and JJC 4-077 [N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)-3-hydroxybutyl)-9H-fluorene-2-carboxamide hydrochloride], and the 2-pyridylphenyl amides, CJB 090 [N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-4-(pyridine-2-yl)benzamide hydrochloride] and PG 01037 [N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)-trans-but-2-enyl)-4-(pyridine-2-yl)benzamide hydrochloride], all of which have been studied in animal models of psychostimulant abuse. Additional screening with a panel of human and rat Supersomes was performed for NGB 2904 and PG 01037. Drug-stimulated ATPase activation assays and bidirectional transport and efflux assays were used to test for substrate specificity of NGB 2904 and PG 01037 for human and rat efflux transporters. All compounds exhibited moderate elimination half-lives, ranging from 1.49 to 3.27 h, and large volumes of distribution (5.95-14.19 l/kg). The brain-to-plasma ratios ranged from 2.93 to 11.81 and were higher than those previously reported for cocaine. Brain exposure levels of NGB 2904 and PG 01037 were significantly reduced after intraperitoneal administration compared with intravenous administration. The metabolism of these compounds was mediated primarily by CYP3A subfamilies. PG 01037 was a P-glycoprotein-transported substrate. Higher doses of these compounds are often required for in vivo action, suggesting decreased bioavailability via extravascular administration that may be attributed to high drug efflux and hepatic metabolism. These studies provide important preclinical information for optimization of next-generation D3R selective agents for the treatment of drug addiction.
Figures




Similar articles
-
Effects of two novel D3-selective compounds, NGB 2904 [N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-9H-fluorene-2-carboxamide] and CJB 090 [N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-4-(pyridin-2-yl)benzamide], on the reinforcing and discriminative stimulus effects of cocaine in rhesus monkeys.J Pharmacol Exp Ther. 2007 May;321(2):573-82. doi: 10.1124/jpet.106.113571. Epub 2007 Feb 1. J Pharmacol Exp Ther. 2007. PMID: 17272677
-
Heterocyclic analogues of N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)arylcarboxamides with functionalized linking chains as novel dopamine D3 receptor ligands: potential substance abuse therapeutic agents.J Med Chem. 2007 Aug 23;50(17):4135-46. doi: 10.1021/jm0704200. Epub 2007 Aug 2. J Med Chem. 2007. PMID: 17672446
-
Influence of cocaine history on the behavioral effects of Dopamine D(3) receptor-selective compounds in monkeys.Neuropsychopharmacology. 2011 Apr;36(5):1104-13. doi: 10.1038/npp.2010.248. Epub 2011 Feb 2. Neuropsychopharmacology. 2011. PMID: 21289600 Free PMC article.
-
Pharmacological actions of NGB 2904, a selective dopamine D3 receptor antagonist, in animal models of drug addiction.CNS Drug Rev. 2007 Summer;13(2):240-59. doi: 10.1111/j.1527-3458.2007.00013.x. CNS Drug Rev. 2007. PMID: 17627675 Free PMC article. Review.
-
Medication discovery for addiction: translating the dopamine D3 receptor hypothesis.Biochem Pharmacol. 2012 Oct 1;84(7):882-90. doi: 10.1016/j.bcp.2012.06.023. Epub 2012 Jul 3. Biochem Pharmacol. 2012. PMID: 22781742 Free PMC article. Review.
Cited by
-
Regulation of human placental drug transporters in HCV infection and their influence on direct acting antiviral medications.Placenta. 2018 Sep;69:32-39. doi: 10.1016/j.placenta.2018.07.005. Epub 2018 Jul 10. Placenta. 2018. PMID: 30213482 Free PMC article.
-
Effect of cyclosporin A on the uptake of D3-selective PET radiotracers in rat brain.Nucl Med Biol. 2011 Jul;38(5):725-39. doi: 10.1016/j.nucmedbio.2011.01.002. Epub 2011 Mar 3. Nucl Med Biol. 2011. PMID: 21718948 Free PMC article.
-
Beyond small-molecule SAR: using the dopamine D3 receptor crystal structure to guide drug design.Adv Pharmacol. 2014;69:267-300. doi: 10.1016/B978-0-12-420118-7.00007-X. Adv Pharmacol. 2014. PMID: 24484980 Free PMC article. Review.
-
The effects of the novel DA D3 receptor antagonist SR 21502 on cocaine reward, cocaine seeking and cocaine-induced locomotor activity in rats.Psychopharmacology (Berl). 2014 Feb;231(3):501-10. doi: 10.1007/s00213-013-3254-y. Epub 2013 Sep 15. Psychopharmacology (Berl). 2014. PMID: 24037509
-
2016 Philip S. Portoghese Medicinal Chemistry Lectureship: Designing Bivalent or Bitopic Molecules for G-Protein Coupled Receptors. The Whole Is Greater Than the Sum of Its Parts.J Med Chem. 2020 Mar 12;63(5):1779-1797. doi: 10.1021/acs.jmedchem.9b01105. Epub 2019 Sep 24. J Med Chem. 2020. PMID: 31499001 Free PMC article.
References
-
- Bailer AJ. (1988) Testing for the equality of area under the curves when using destructive measurement techniques. J Pharmacokinet Biopharm 16:303–309 - PubMed
-
- Boeckler F, Gmeiner P. (2006) The structural evolution of dopamine D3 receptor ligands: structure–activity relationships and selected neuropharmacological aspects. Pharmacol Ther 112:281–333 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

