Long-term ritonavir exposure increases fatty acid and glycerol recycling in 3T3-L1 adipocytes as compensatory mechanisms for increased triacylglycerol hydrolysis
- PMID: 20228169
- PMCID: PMC2869264
- DOI: 10.1210/en.2009-1364
Long-term ritonavir exposure increases fatty acid and glycerol recycling in 3T3-L1 adipocytes as compensatory mechanisms for increased triacylglycerol hydrolysis
Abstract
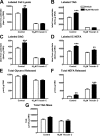
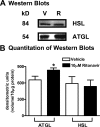
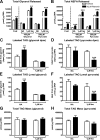
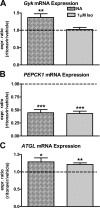
Lipodystrophy with high nonesterified fatty acid (FA) efflux is reported in humans receiving highly active antiretroviral therapy (HAART) to treat HIV infection. Ritonavir, a common component of HAART, alters adipocyte FA efflux, but the mechanism for this effect is not established. To investigate ritonavir-induced changes in FA flux and recycling through acylglycerols, we exposed differentiated murine 3T3-L1 adipocytes to ritonavir for 14 d. FA efflux, uptake, and incorporation into acylglycerols were measured. To identify a mediator of FA efflux, we measured adipocyte triacylglycerol lipase (ATGL) transcript and protein. To determine whether ritonavir-treated adipocytes increased glycerol backbone synthesis for FA reesterification, we measured labeled glycerol and pyruvate incorporation into triacylglycerol (TAG). Ritonavir-treated cells had increased FA efflux, uptake, and incorporation into TAG (all P < 0.01). Ritonavir increased FA efflux without consistently increasing glycerol release or changing TAG mass, suggesting increased partial TAG hydrolysis. Ritonavir-treated adipocytes expressed significantly more ATGL mRNA (P < 0.05) and protein (P < 0.05). Ritonavir increased glycerol (P < 0.01) but not pyruvate (P = 0.41), utilization for TAG backbone synthesis. Consistent with this substrate utilization, glycerol kinase transcript (required for glycerol incorporation into TAG backbone) was up-regulated (P < 0.01), whereas phosphoenolpyruvate carboxykinase transcript (required for pyruvate utilization) was down-regulated (P < 0.001). In 3T3-L1 adipocytes, long-term ritonavir exposure perturbs FA metabolism by increasing ATGL-mediated partial TAG hydrolysis, thus increasing FA efflux, and leads to compensatory increases in FA reesterification with glycerol and acylglycerols. These changes in FA metabolism may, in part, explain the increased FA efflux observed in ritonavir-associated lipodystrophy.
Figures
Similar articles
-
1,25-Dihydroxyvitamin D regulates lipid metabolism and glucose utilization in differentiated 3T3-L1 adipocytes.Nutr Res. 2018 Oct;58:72-83. doi: 10.1016/j.nutres.2018.07.004. Epub 2018 Jul 12. Nutr Res. 2018. PMID: 30340817
-
Ritonavir increases the level of active ADD-1/SREBP-1 protein during adipogenesis.AIDS. 2000 Nov 10;14(16):2467-73. doi: 10.1097/00002030-200011100-00007. AIDS. 2000. PMID: 11101056
-
Distinct long-term regulation of glycerol and non-esterified fatty acid release by insulin and TNF-alpha in 3T3-L1 adipocytes.Diabetologia. 2001 Jan;44(1):55-62. doi: 10.1007/s001250051580. Diabetologia. 2001. PMID: 11206412
-
Glucose enhances catecholamine-stimulated lipolysis via increased glycerol-3-phosphate synthesis in 3T3-L1 adipocytes and rat adipose tissue.Mol Biol Rep. 2021 Sep;48(9):6269-6276. doi: 10.1007/s11033-021-06617-1. Epub 2021 Aug 10. Mol Biol Rep. 2021. PMID: 34374898
-
Fatty acid recycling in adipocytes: a role for glyceroneogenesis and phosphoenolpyruvate carboxykinase.Biochem Soc Trans. 2003 Dec;31(Pt 6):1125-9. doi: 10.1042/bst0311125. Biochem Soc Trans. 2003. PMID: 14641009 Review.
Cited by
-
Toll-like receptor agonists promote prolonged triglyceride storage in macrophages.J Biol Chem. 2014 Jan 31;289(5):3001-12. doi: 10.1074/jbc.M113.524587. Epub 2013 Dec 11. J Biol Chem. 2014. PMID: 24337578 Free PMC article.
-
Body fat redistribution and metabolic abnormalities in HIV-infected patients on highly active antiretroviral therapy: novel insights into pathophysiology and emerging opportunities for treatment.Metabolism. 2011 Jun;60(6):749-53. doi: 10.1016/j.metabol.2010.09.011. Epub 2010 Oct 20. Metabolism. 2011. PMID: 20965525 Free PMC article. No abstract available.
-
Glyceroneogenesis is inhibited through HIV protease inhibitor-induced inflammation in human subcutaneous but not visceral adipose tissue.J Lipid Res. 2011 Feb;52(2):207-20. doi: 10.1194/jlr.M000869. Epub 2010 Nov 10. J Lipid Res. 2011. PMID: 21068005 Free PMC article.
-
Comparison of in vitro and in situ methods for studying lipolysis.ISRN Endocrinol. 2013 Aug 19;2013:205385. doi: 10.1155/2013/205385. ISRN Endocrinol. 2013. PMID: 24024037 Free PMC article. Review.
-
Proteomic profiling of adipose tissue from Zmpste24-/- mice, a model of lipodystrophy and premature aging, reveals major changes in mitochondrial function and vimentin processing.Mol Cell Proteomics. 2011 Nov;10(11):M111.008094. doi: 10.1074/mcp.M111.008094. Epub 2011 Aug 9. Mol Cell Proteomics. 2011. PMID: 21828285 Free PMC article.
References
-
- Cadoudal T, Leroyer S, Reis AF, Tordjman J, Durant S, Fouque F, Collinet M, Quette J, Chauvet G, Beale E, Velho G, Antoine B, Benelli C, Forest C 2005 Proposed involvement of adipocyte glyceroneogenesis and phosphoenolpyruvate carboxykinase in the metabolic syndrome. Biochimie (Paris) 87:27–32 - PubMed
-
- Prentki M, Madiraju SR 2008 Glycerolipid metabolism and signaling in health and disease. Endocr Rev 29:647–676 - PubMed
-
- Arner P 2005 Human fat cell lipolysis: biochemistry, regulation and clinical role. Best Pract Res Clin Endocrinol Metab 19:471–482 - PubMed
-
- Kratz M, Purnell JQ, Breen PA, Thomas KK, Utzschneider KM, Carr DB, Kahn SE, Hughes JP, Rutledge EA, Van Yserloo B, Yukawa M, Weigle DS 2008 Reduced adipogenic gene expression in thigh adipose tissue precedes human immunodeficiency virus-associated lipoatrophy. J Clin Endocrinol Metab 93:959–966 - PMC - PubMed
-
- Mallon PW 2007 Pathogenesis of lipodystrophy and lipid abnormalities in patients taking antiretroviral therapy. AIDS Rev 9:3–15 - PubMed

