Direct action through the sertoli cells is essential for androgen stimulation of spermatogenesis
- PMID: 20228170
- PMCID: PMC2871953
- DOI: 10.1210/en.2009-1333
Direct action through the sertoli cells is essential for androgen stimulation of spermatogenesis
Abstract
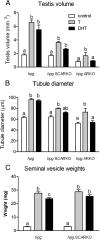
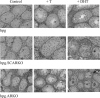
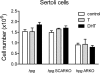
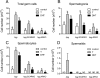
Androgens act to stimulate spermatogenesis through androgen receptors (ARs) on the Sertoli cells and peritubular myoid cells. Specific ablation of the AR in either cell type will cause a severe disruption of spermatogenesis. To determine whether androgens can stimulate spermatogenesis through direct action on the peritubular myoid cells alone or whether action on the Sertoli cells is essential, we crossed hypogonadal (hpg) mice that lack gonadotrophins and intratesticular androgen with mice lacking ARs either ubiquitously (ARKO) or specifically on the Sertoli cells (SCARKO). These hpg.ARKO and hpg.SCARKO mice were treated with testosterone (T) or dihydrotestosterone (DHT) for 7 d and testicular morphology and cell numbers assessed. Androgen treatment did not affect Sertoli cell numbers in any animal group. Both T and DHT increased numbers of spermatogonia and spermatocytes in hpg mice, but DHT has no effect on germ cell numbers in hpg.SCARKO and hpg.ARKO mice. T increased germ cell numbers in hpg.SCARKO and hpg.ARKO mice, but this was associated with stimulation of FSH release. Results show that androgen stimulation of spermatogenesis requires direct androgen action on the Sertoli cells.
Figures


Similar articles
-
Effect of FSH on testicular morphology and spermatogenesis in gonadotrophin-deficient hypogonadal mice lacking androgen receptors.Reproduction. 2010 Jan;139(1):177-84. doi: 10.1530/REP-09-0377. Reproduction. 2010. PMID: 19846485 Free PMC article.
-
Testicular development in mice lacking receptors for follicle stimulating hormone and androgen.PLoS One. 2012;7(4):e35136. doi: 10.1371/journal.pone.0035136. Epub 2012 Apr 13. PLoS One. 2012. PMID: 22514715 Free PMC article.
-
Occurrence of testicular microlithiasis in androgen insensitive hypogonadal mice.Reprod Biol Endocrinol. 2009 Aug 27;7:88. doi: 10.1186/1477-7827-7-88. Reprod Biol Endocrinol. 2009. PMID: 19712470 Free PMC article.
-
Regulation of seminiferous tubular function by FSH and androgen.J Reprod Fertil. 1975 Aug;44(2):363-75. doi: 10.1530/jrf.0.0440363. J Reprod Fertil. 1975. PMID: 169349 Review.
-
Hormonal control of germ cell development and spermatogenesis.Semin Cell Dev Biol. 2014 May;29:55-65. doi: 10.1016/j.semcdb.2014.02.010. Epub 2014 Mar 2. Semin Cell Dev Biol. 2014. PMID: 24598767 Review.
Cited by
-
Possible therapeutic use of spermatogonial stem cells in the treatment of male infertility: a brief overview.ScientificWorldJournal. 2012;2012:374151. doi: 10.1100/2012/374151. Epub 2012 Mar 12. ScientificWorldJournal. 2012. PMID: 22536138 Free PMC article. Review.
-
Peritubular myoid cells participate in male mouse spermatogonial stem cell maintenance.Endocrinology. 2014 Dec;155(12):4964-74. doi: 10.1210/en.2014-1406. Epub 2014 Sep 2. Endocrinology. 2014. PMID: 25181385 Free PMC article.
-
Functions of Steroid Hormones in the Male Reproductive Tract as Revealed by Mouse Models.Int J Mol Sci. 2023 Feb 1;24(3):2748. doi: 10.3390/ijms24032748. Int J Mol Sci. 2023. PMID: 36769069 Free PMC article. Review.
-
Androgen receptor expression is required to ensure development of adult Leydig cells and to prevent development of steroidogenic cells with adrenal characteristics in the mouse testis.BMC Dev Biol. 2019 Apr 17;19(1):8. doi: 10.1186/s12861-019-0189-5. BMC Dev Biol. 2019. PMID: 30995907 Free PMC article.
-
scRNA-Seq-Based Transcriptome Profiling and Relevant Bioinformatics Approaches to Uncover Novel Insights in Studying Human Spermatogenesis.Adv Exp Med Biol. 2025;1469:173-205. doi: 10.1007/978-3-031-82990-1_9. Adv Exp Med Biol. 2025. PMID: 40301258 Review.
References
-
- Lyon MF, Hawkes SG 1970 X-linked gene for testicular feminization in the mouse. Nature 227:1217–1219 - PubMed
-
- De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lécureuil C, Heyns W, Carmeliet P, Guillou F, Sharpe RM, Verhoeven G 2004 A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci USA 101:1327–1332 - PMC - PubMed
-
- Abel MH, Wootton AN, Wilkins V, Huhtaniemi I, Knight PG, Charlton HM 2000 The effect of a null mutation in the follicle-stimulating hormone receptor gene on mouse reproduction. Endocrinology 141:1795–1803 - PubMed
-
- Kumar TR, Wang Y, Lu N, Matzuk MM 1997 Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet 15:201–204 - PubMed
-
- Krishnamurthy H, Danilovich N, Morales CR, Sairam MR 2000 Qualitative and quantitative decline in spermatogenesis of the follicle-stimulating hormone receptor knockout (FORKO) mouse. Biol Reprod 62:1146–1159 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

