Measurement and analysis of sarcomere length in rat cardiomyocytes in situ and in vitro
- PMID: 20228259
- PMCID: PMC2867435
- DOI: 10.1152/ajpheart.00481.2009
Measurement and analysis of sarcomere length in rat cardiomyocytes in situ and in vitro
Abstract
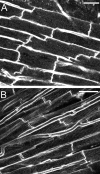
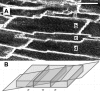
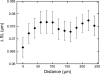
Sarcomere length (SL) is an important determinant and indicator of cardiac mechanical function; however, techniques for measuring SL in living, intact tissue are limited. Here, we present a technique that uses two-photon microscopy to directly image striations of living cells in cardioplegic conditions, both in situ (Langendorff-perfused rat hearts and ventricular tissue slices, stained with the fluorescent marker di-4-ANEPPS) and in vitro (acutely isolated rat ventricular myocytes). Software was developed to extract SL from two-photon fluorescence image sets while accounting for measurement errors associated with motion artifact in raster-scanned images and uncertainty of the cell angle relative to the imaging plane. Monte-Carlo simulations were used to guide analysis of SL measurements by determining error bounds as a function of measurement path length. The mode of the distribution of SL measurements in resting Langendorff-perfused heart is 1.95 mum (n = 167 measurements from N = 11 hearts) after correction for tissue orientation, which was significantly greater than that in isolated cells (1.71 mum, n = 346, N = 9 isolations) or ventricular slice preparations (1.79 mum, n = 79, N = 3 hearts) under our experimental conditions. Furthermore, we find that edema in arrested Langendorff-perfused heart is associated with a mean SL increase; this occurs as a function of time ex vivo and correlates with tissue volume changes determined by magnetic resonance imaging. Our results highlight that the proposed method can be used to monitor SL in living cells and that different experimental models from the same species may display significantly different SL values under otherwise comparable conditions, which has implications for experiment design, as well as comparison and interpretation of data.
Figures




References
-
- Aistrup GL, Shiferaw Y, Kapur S, Kadish A, Wasserstrom JA. Mechanism underlying the formation and dynamics of subcellular calcium alternans in the intact rat heart. Circ Res 104: 639–649, 2009 - PubMed
-
- Bassien-Capsa V, Fouron JC, Comte B, Chorvatova A. Structural, functional and metabolic remodeling of rat left ventricular myocytes in normal and in sodium-supplemented pregnancy. Cardiovasc Res 69: 423–431, 2006 - PubMed
-
- Bers DM. Ca influx and sarcoplasmic reticulum Ca release in cardiac muscle activation during postrest recovery. Am J Physiol Heart Circ Physiol 248: H366–H381, 1985 - PubMed
-
- Boulesteix T, Beaurepaire E, Sauviat MP, Schanne-Klein MC. Second-harmonic microscopy of unstained living cardiac myocytes: measurements of sarcomere length with 20-nm accuracy. Opt Lett 29: 2031–2033, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

