Dysregulation of promyelocytic leukemia (PML) protein expression in preeclamptic placentae
- PMID: 20228380
- PMCID: PMC2857389
- DOI: 10.1177/1933719109358455
Dysregulation of promyelocytic leukemia (PML) protein expression in preeclamptic placentae
Abstract
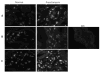
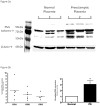
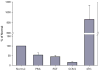
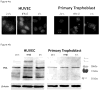
Promyelocytic leukemia (PML) protein is a nucleoprotein that can regulate a variety of cellular stress responses. The aim of this study was to determine qualitative and quantitative changes in PML expression in preeclamptic placentae. Immunoblot, quantitative reverse transcription polymerase chain reaction (qRT-PCR), and immunohistochemistry techniques were used to determine PML gene expression and localization in normal (n = 6) and preeclamptic (n = 6) placentae and primary cells. Promyelocytic leukemia protein was immunolocalized within nuclei of villus mesenchyme, but largely absent in trophoblast nuclei, with a trend for increased PML reactivity in preeclamptic placenta. Immunoblot analyses of nuclear extracts confirmed relative increases (approximately 3-fold) of PML expression in preeclamptic placentae (P < .05). Conversely, less PML messenger RNA (mRNA; approximately 2-fold) was detected in preeclamptic versus normal placental samples. In vitro, PML expression could be increased by hypoxia in cultured endothelial cells but not trophoblast. Increased PML protein expression in preeclamptic villi suggests it could contribute to decreased vascularity and placental growth and/or function.
Figures
Similar articles
-
Trophoblast debris extruded from preeclamptic placentae activates endothelial cells: a mechanism by which the placenta communicates with the maternal endothelium.Placenta. 2014 Oct;35(10):839-47. doi: 10.1016/j.placenta.2014.07.009. Epub 2014 Jul 24. Placenta. 2014. PMID: 25096950
-
Reduced expression of survivin, the inhibitor of apoptosis protein correlates with severity of preeclampsia.Placenta. 2012 Jan;33(1):47-51. doi: 10.1016/j.placenta.2011.10.008. Epub 2011 Oct 26. Placenta. 2012. PMID: 22033156
-
Promyelocytic leukaemia (PML) protein expression in human placenta and choriocarcinoma.J Pathol. 2003 Sep;201(1):83-9. doi: 10.1002/path.1382. J Pathol. 2003. PMID: 12950020
-
Expression of von Hippel Lindau (pVHL) protein in placentae from normal pregnant women and women with preeclampsia.Placenta. 2006 Apr-May;27(4-5):411-21. doi: 10.1016/j.placenta.2005.04.002. Epub 2005 Jun 13. Placenta. 2006. PMID: 15955559
-
PML tumour suppression and beyond: therapeutic implications.FEBS Lett. 2014 Aug 19;588(16):2653-62. doi: 10.1016/j.febslet.2014.02.007. Epub 2014 Feb 15. FEBS Lett. 2014. PMID: 24548562 Review.
References
-
- ACOG. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 2002;77:67–75. - PubMed
-
- Parra M, Rodrigo R, Barja P, Bosco C, Fernandez V, Munoz H, et al. Screening test for preeclampsia through assessment of uteroplacental blood flow and biochemical markers of oxidative stress and endothelial dysfunction. Am J Obstet Gynecol. 2005;193:1486–91. - PubMed
-
- Redman CW, Sargent IL. The pathogenesis of pre-eclampsia. Gynecol Obstet Fertil. 2001;29:518–22. - PubMed
-
- Roberts JM. Endothelial dysfunction in preeclampsia. Semin Reprod Endocrinol. 1998;16:5–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

