Cytotoxic substances from two species of Japanese sea hares: chemistry and bioactivity
- PMID: 20228619
- PMCID: PMC3417844
- DOI: 10.2183/pjab.86.176
Cytotoxic substances from two species of Japanese sea hares: chemistry and bioactivity
Abstract
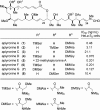
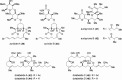
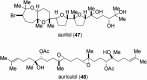
From the sea hares belonging to two genera, Aplysia and Dolabella, a variety of new cytotoxic substances were isolated as minute constituents: their chemical structures were determined and their cytotoxicity was evaluated. Regarding the highly cytotoxic substances, further chemical and biological studies were performed that included their asymmetric chemical synthesis and elucidation of biological characteristics such as antitumor activity.
Figures

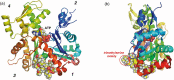




Similar articles
-
The cyanobacterial origin of potent anticancer agents originally isolated from sea hares.Curr Med Chem. 2002 Oct;9(20):1791-806. doi: 10.2174/0929867023369051. Curr Med Chem. 2002. PMID: 12369878 Review.
-
Cytotoxic sesquiterpenes from Aplysia dactylomela.J Nat Prod. 2005 Nov;68(11):1677-9. doi: 10.1021/np050240y. J Nat Prod. 2005. PMID: 16309323
-
Cytotoxic screening of medicinal and edible plants in Okinawa, Japan, and identification of the main toxic constituent of Rhodea japonica (Omoto).Biosci Biotechnol Biochem. 2003 Jun;67(6):1401-4. doi: 10.1271/bbb.67.1401. Biosci Biotechnol Biochem. 2003. PMID: 12843672
-
Aplaminal: a novel cytotoxic aminal isolated from the sea hare Aplysia kurodai.Org Lett. 2008 Feb 7;10(3):489-91. doi: 10.1021/ol7029307. Epub 2008 Jan 10. Org Lett. 2008. PMID: 18184001
-
Bioactive molecules from sea hares.Prog Mol Subcell Biol. 2006;43:215-39. doi: 10.1007/978-3-540-30880-5_10. Prog Mol Subcell Biol. 2006. PMID: 17153345 Review.
Cited by
-
Targeting and extending the eukaryotic druggable genome with natural products: cytoskeletal targets of natural products.Nat Prod Rep. 2020 May 1;37(5):634-652. doi: 10.1039/c9np00053d. Epub 2019 Nov 25. Nat Prod Rep. 2020. PMID: 31764930 Free PMC article. Review.
-
Bioactive Compounds from Marine Heterobranchs.Mar Drugs. 2020 Dec 21;18(12):657. doi: 10.3390/md18120657. Mar Drugs. 2020. PMID: 33371188 Free PMC article. Review.
-
Chemical Diversity and Biological Properties of Secondary Metabolites from Sea Hares of Aplysia Genus.Mar Drugs. 2016 Feb 19;14(2):39. doi: 10.3390/md14020039. Mar Drugs. 2016. PMID: 26907303 Free PMC article. Review.
-
Bioactive substances with anti-neoplastic efficacy from marine invertebrates: Bryozoa, Mollusca, Echinodermata and Urochordata.World J Clin Oncol. 2011 Nov 10;2(11):362-6. doi: 10.5306/wjco.v2.i11.362. World J Clin Oncol. 2011. PMID: 22087434 Free PMC article.
-
Synthesis and Biological Activities of Aplyronine A Analogues toward the Development of Antitumor Protein-Protein Interaction Inducers between Actin and Tubulin: Conjugation of the C1-C9 Macrolactone Part and the C24-C34 Side Chain.ACS Omega. 2019 May 16;4(5):8598-8613. doi: 10.1021/acsomega.9b01099. eCollection 2019 May 31. ACS Omega. 2019. PMID: 31459949 Free PMC article.
References
-
- Blunt J.W., Copp B.R., Hu W.P., Munro M.H.G., Northcote P.T., Princep M.R. (2009) Marine natural products. Nat. Prod. Rep. 26, 170–244 - PubMed
-
- Harrigan G.G., Goetz G. (2002) Symbiotic and dietary marine microalgae as a source of bioactive molecules-experience from natural products research. J. Appl. Phycol. 14, 103–108
-
- Yamamura S., Hirata Y. (1963) Structure of aplysin and aplysinol, naturally occurring bromo compounds. Tetrahedron 19, 1485–1496
-
- Pettit G.R. (1997) The dolastatins. InProgress in the Chemistry of Organic Natural Products. Vol. 70 (eds. Herz W., Kirby G.W., Moore R.E., Steglich W., Tamm Ch.). Springer, Wien/New York, pp. 1–79 - PubMed
-
- Yamada K., Kigoshi H. (1997) Bioactive compounds from the sea hares of two genera: Aplysia and Dolabella. Bull. Chem. Soc. Jpn. 70, 1479–1489

