Autophagy and the ubiquitin-proteasome system in cardiac dysfunction
- PMID: 20228723
- PMCID: PMC2840262
Autophagy and the ubiquitin-proteasome system in cardiac dysfunction
Abstract
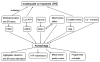
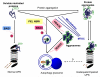
The ubiquitin-proteasome system (UPS) and autophagy are two major intracellular protein degradation pathways. The UPS mediates the removal of soluble abnormal proteins as well as the targeted degradation of most normal proteins that are no longer needed. Autophagy is generally responsible for bulky removal of defective organelles and for sequestering portions of cytoplasm for lysosomal degradation during starvation. Impaired or inadequate protein degradation in the heart is associated with and may be a major pathogenic factor for a wide variety of cardiac dysfunctions, while enhanced protein degradation is also implicated in the development of cardiac pathology. It was generally assumed that the UPS and autophagy serve distinct functions. Therefore, the functional roles of the UPS and autophagy in the hearts have been largely investigated separately. However, recent advances in understanding the shared mechanisms contributing to UPS alteration and the induction of autophagy have helped reveal the link and interplay between the two proteolytic systems in the heart. These links are exemplified by scenarios in which inadequate UPS proteolytic function leads to activation of autophagy, helping alleviate proteotoxic stress. It is becoming increasingly clear that a coordinated and complementary relationship between the two systems is critical to protect cells against stress. Several proteins including p62, NBR1, HDAC6, and co-chaperones appear to play an important role in harmonizing and mobilizing the consortium formed by the UPS and autophagy.
Figures
Similar articles
-
Interplay between the ubiquitin-proteasome system and autophagy in proteinopathies.Int J Physiol Pathophysiol Pharmacol. 2009 May 8;1(2):127-42. Int J Physiol Pathophysiol Pharmacol. 2009. PMID: 20411034 Free PMC article.
-
The interplay between autophagy and the ubiquitin-proteasome system in cardiac proteotoxicity.Biochim Biophys Acta. 2015 Feb;1852(2):188-94. doi: 10.1016/j.bbadis.2014.07.028. Epub 2014 Aug 1. Biochim Biophys Acta. 2015. PMID: 25092168 Free PMC article. Review.
-
HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS.Nature. 2007 Jun 14;447(7146):859-63. doi: 10.1038/nature05853. Nature. 2007. PMID: 17568747
-
The ubiquitin-proteasome system in cardiac dysfunction.Biochim Biophys Acta. 2008 Dec;1782(12):749-63. doi: 10.1016/j.bbadis.2008.06.009. Epub 2008 Jun 25. Biochim Biophys Acta. 2008. PMID: 18634872 Review.
-
p62 Stages an interplay between the ubiquitin-proteasome system and autophagy in the heart of defense against proteotoxic stress.Trends Cardiovasc Med. 2011 Nov;21(8):224-8. doi: 10.1016/j.tcm.2012.05.015. Trends Cardiovasc Med. 2011. PMID: 22902070 Free PMC article. Review.
Cited by
-
A Transgenic Mouse Model of Eccentric Left Ventricular Hypertrophy With Preserved Ejection Fraction Exhibits Alterations in the Autophagy-Lysosomal Pathway.Front Physiol. 2021 Apr 22;12:614878. doi: 10.3389/fphys.2021.614878. eCollection 2021. Front Physiol. 2021. PMID: 33995116 Free PMC article.
-
Remote Ischemic Preconditioning induces Cardioprotective Autophagy and Signals through the IL-6-Dependent JAK-STAT Pathway.Int J Mol Sci. 2020 Mar 1;21(5):1692. doi: 10.3390/ijms21051692. Int J Mol Sci. 2020. PMID: 32121587 Free PMC article.
-
The role of the proteasome in heart disease.Biochim Biophys Acta. 2011 Feb;1809(2):141-9. doi: 10.1016/j.bbagrm.2010.09.001. Epub 2010 Sep 15. Biochim Biophys Acta. 2011. PMID: 20840877 Free PMC article. Review.
-
Cardiac systolic dysfunction in doxorubicin-challenged rats is associated with upregulation of MuRF2 and MuRF3 E3 ligases.Exp Clin Cardiol. 2012 Sep;17(3):101-9. Exp Clin Cardiol. 2012. PMID: 23620696 Free PMC article.
-
The ubiquitin proteasome system and myocardial ischemia.Am J Physiol Heart Circ Physiol. 2013 Feb 1;304(3):H337-49. doi: 10.1152/ajpheart.00604.2012. Epub 2012 Dec 7. Am J Physiol Heart Circ Physiol. 2013. PMID: 23220331 Free PMC article. Review.
References
-
- Fuertes G, Villarroya A, Knecht E. Role of proteasomes in the degradation of short-lived proteins in human fibroblasts under various growth conditions. Int J Biochem Cell Biol. 2003;35:651–664. - PubMed
-
- Hochstrasser M. Ubiquitin, proteasomes, and the regulation of intracellular protein degradation. Curr Opin Cell Biol. 1995;7:215–223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

