Molecular control of kinetochore-microtubule dynamics and chromosome oscillations
- PMID: 20228811
- PMCID: PMC2909587
- DOI: 10.1038/ncb2033
Molecular control of kinetochore-microtubule dynamics and chromosome oscillations
Abstract
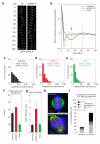
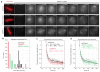
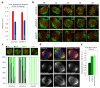
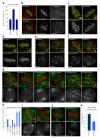
Chromosome segregation in metazoans requires the alignment of sister kinetochores on the metaphase plate. During chromosome alignment, bioriented kinetochores move chromosomes by regulating the plus-end dynamics of the attached microtubules. The bundles of kinetochore-bound microtubules alternate between growth and shrinkage, leading to regular oscillations along the spindle axis. However, the molecular mechanisms that coordinate microtubule plus-end dynamics remain unknown. Here we show that centromere protein (CENP)-H, a subunit of the CENP-A nucleosome-associated and CENP-A distal complexes (CENP-A NAC/CAD), is essential for this coordination, because kinetochores lacking CENP-H establish bioriented attachments but fail to generate regular oscillations, as a result of an uncontrolled rate of microtubule plus-end turnover. These alterations lead to rapid erratic movements that disrupt metaphase plate organization. We also show that the abundance of the CENP-A NAC/CAD subunits CENP-H and CENP-I dynamically change on individual sister kinetochores in vivo, because they preferentially bind the sister kinetochore attached to growing microtubules, and that one other subunit, CENP-Q, binds microtubules in vitro. We therefore propose that CENP-A NAC/CAD is a direct regulator of kinetochore-microtubule dynamics, which physically links centromeric DNA to microtubule plus ends.
Figures


Similar articles
-
CENP-E kinesin interacts with SKAP protein to orchestrate accurate chromosome segregation in mitosis.J Biol Chem. 2012 Jan 6;287(2):1500-9. doi: 10.1074/jbc.M111.277194. Epub 2011 Nov 22. J Biol Chem. 2012. PMID: 22110139 Free PMC article.
-
Mitotic Protein CSPP1 Interacts with CENP-H Protein to Coordinate Accurate Chromosome Oscillation in Mitosis.J Biol Chem. 2015 Nov 6;290(45):27053-27066. doi: 10.1074/jbc.M115.658534. Epub 2015 Sep 16. J Biol Chem. 2015. PMID: 26378239 Free PMC article.
-
Septin 7 interacts with centromere-associated protein E and is required for its kinetochore localization.J Biol Chem. 2008 Jul 4;283(27):18916-25. doi: 10.1074/jbc.M710591200. Epub 2008 May 6. J Biol Chem. 2008. PMID: 18460473 Free PMC article.
-
Mechanisms of kinesin-7 CENP-E in kinetochore-microtubule capture and chromosome alignment during cell division.Biol Cell. 2019 Jun;111(6):143-160. doi: 10.1111/boc.201800082. Epub 2019 Feb 26. Biol Cell. 2019. PMID: 30784092 Review.
-
The unconventional kinetoplastid kinetochore: from discovery toward functional understanding.Biochem Soc Trans. 2016 Oct 15;44(5):1201-1217. doi: 10.1042/BST20160112. Biochem Soc Trans. 2016. PMID: 27911702 Free PMC article. Review.
Cited by
-
The CENP-T C-terminus is exclusively proximal to H3.1 and not to H3.2 or H3.3.Int J Mol Sci. 2015 Mar 12;16(3):5839-63. doi: 10.3390/ijms16035839. Int J Mol Sci. 2015. PMID: 25775162 Free PMC article.
-
Polar Chromosomes-Challenges of a Risky Path.Cells. 2022 May 3;11(9):1531. doi: 10.3390/cells11091531. Cells. 2022. PMID: 35563837 Free PMC article. Review.
-
K-fibre minus ends are stabilized by a RanGTP-dependent mechanism essential for functional spindle assembly.Nat Cell Biol. 2011 Nov 13;13(12):1406-14. doi: 10.1038/ncb2372. Nat Cell Biol. 2011. PMID: 22081094
-
Fission yeast kinesin-8 controls chromosome congression independently of oscillations.J Cell Sci. 2015 Oct 15;128(20):3720-30. doi: 10.1242/jcs.160465. Epub 2015 Sep 10. J Cell Sci. 2015. PMID: 26359299 Free PMC article.
-
Distinct Roles of the Chromosomal Passenger Complex in the Detection of and Response to Errors in Kinetochore-Microtubule Attachment.Dev Cell. 2017 Sep 25;42(6):640-654.e5. doi: 10.1016/j.devcel.2017.08.022. Dev Cell. 2017. PMID: 28950102 Free PMC article.
References
-
- Cheeseman IM, Chappie JS, Wilson-Kubalek EM, Desai A. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell. 2006;127:983–997. - PubMed
-
- Lewis WH. Changes of viscosity and cell activity. Science. 1939;89:400.
-
- Hughes AF, Swann MM. Anaphase movements in the living cell. J. Exp. Biol. 1948;25:45–70.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

