Tumor cell-specific bioluminescence platform to identify stroma-induced changes to anticancer drug activity
- PMID: 20228816
- PMCID: PMC3786785
- DOI: 10.1038/nm.2112
Tumor cell-specific bioluminescence platform to identify stroma-induced changes to anticancer drug activity
Erratum in
-
Author Correction: Tumor cell-specific bioluminescence platform to identify stroma-induced changes to anticancer drug activity.Nat Med. 2024 Apr;30(4):1214. doi: 10.1038/s41591-024-02847-5. Nat Med. 2024. PMID: 38355975 No abstract available.
Abstract
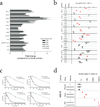
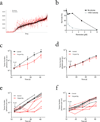
Conventional anticancer drug screening is typically performed in the absence of accessory cells of the tumor microenvironment, which can profoundly alter antitumor drug activity. To address this limitation, we developed the tumor cell-specific in vitro bioluminescence imaging (CS-BLI) assay. Tumor cells (for example, myeloma, leukemia and solid tumors) stably expressing luciferase are cultured with nonmalignant accessory cells (for example, stromal cells) for selective quantification of tumor cell viability, in presence versus absence of stromal cells or drug treatment. CS-BLI is high-throughput scalable and identifies stroma-induced chemoresistance in diverse malignancies, including imatinib resistance in leukemic cells. A stroma-induced signature in tumor cells correlates with adverse clinical prognosis and includes signatures for activated Akt, Ras, NF-kappaB, HIF-1alpha, myc, hTERT and IRF4; for biological aggressiveness; and for self-renewal. Unlike conventional screening, CS-BLI can also identify agents with increased activity against tumor cells interacting with stroma. One such compound, reversine, shows more potent activity in an orthotopic model of diffuse myeloma bone lesions than in conventional subcutaneous xenografts. Use of CS-BLI, therefore, enables refined screening of candidate anticancer agents to enrich preclinical pipelines with potential therapeutics that overcome stroma-mediated drug resistance and can act in a synthetic lethal manner in the context of tumor-stroma interactions.
Figures


Similar articles
-
The role of tumour-stromal interactions in modifying drug response: challenges and opportunities.Nat Rev Drug Discov. 2013 Mar;12(3):217-28. doi: 10.1038/nrd3870. Nat Rev Drug Discov. 2013. PMID: 23449307 Review.
-
Fluorescence-based experimental model to evaluate the concomitant effect of drugs on the tumour microenvironment and cancer cells.Br J Haematol. 2012 Jun;157(5):564-79. doi: 10.1111/j.1365-2141.2012.09103.x. Epub 2012 Mar 20. Br J Haematol. 2012. PMID: 22428569
-
A method for measurement of drug sensitivity of myeloma cells co-cultured with bone marrow stromal cells.J Biomol Screen. 2013 Jul;18(6):637-46. doi: 10.1177/1087057113478168. Epub 2013 Feb 27. J Biomol Screen. 2013. PMID: 23446700
-
Compartment-Specific Bioluminescence Imaging platform for the high-throughput evaluation of antitumor immune function.Blood. 2012 Apr 12;119(15):e131-8. doi: 10.1182/blood-2011-04-348490. Epub 2012 Jan 30. Blood. 2012. PMID: 22289890 Free PMC article.
-
The critical role of the bone marrow stromal microenvironment for the development of drug screening platforms in leukemia.Exp Hematol. 2024 May;133:104212. doi: 10.1016/j.exphem.2024.104212. Epub 2024 Mar 28. Exp Hematol. 2024. PMID: 38552942 Review.
Cited by
-
Aberrant regulation of the BST2 (Tetherin) promoter enhances cell proliferation and apoptosis evasion in high grade breast cancer cells.PLoS One. 2013 Jun 20;8(6):e67191. doi: 10.1371/journal.pone.0067191. Print 2013. PLoS One. 2013. PMID: 23840623 Free PMC article.
-
The role of tumour-stromal interactions in modifying drug response: challenges and opportunities.Nat Rev Drug Discov. 2013 Mar;12(3):217-28. doi: 10.1038/nrd3870. Nat Rev Drug Discov. 2013. PMID: 23449307 Review.
-
Increased drug resistance in breast cancer by tumor-associated macrophages through IL-10/STAT3/bcl-2 signaling pathway.Med Oncol. 2015 Feb;32(2):352. doi: 10.1007/s12032-014-0352-6. Epub 2015 Jan 9. Med Oncol. 2015. PMID: 25572805
-
High-throughput approaches to discover novel immunomodulatory agents for cancer.Oncoimmunology. 2012 Nov 1;1(8):1406-1408. doi: 10.4161/onci.21058. Oncoimmunology. 2012. PMID: 23243609 Free PMC article.
-
Stromal CYR61 Confers Resistance to Mitoxantrone via Spleen Tyrosine Kinase Activation in Human Acute Myeloid Leukaemia.Br J Haematol. 2015 Sep;170(5):704-18. doi: 10.1111/bjh.13492. Epub 2015 May 14. Br J Haematol. 2015. PMID: 25974135 Free PMC article. Clinical Trial.
References
-
- Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. - PubMed
-
- Karnoub AE, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. - PubMed
-
- Mitsiades CS, Mitsiades N, Munshi NC, Anderson KC. Focus on multiple myeloma. Cancer Cell. 2004;6:439–444. - PubMed
-
- Grigorieva I, Thomas X, Epstein J. The bone marrow stromal environment is a major factor in myeloma cell resistance to dexamethasone. Exp Hematol. 1998;26:597–603. - PubMed
-
- Hurt EM, et al. Overexpression of c-maf is a frequent oncogenic event in multiple myeloma that promotes proliferation and pathological interactions with bone marrow stroma. Cancer Cell. 2004;5:191–199. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

