Protein kinase Cvarepsilon mediates Stat3Ser727 phosphorylation, Stat3-regulated gene expression, and cell invasion in various human cancer cell lines through integration with MAPK cascade (RAF-1, MEK1/2, and ERK1/2)
- PMID: 20228845
- PMCID: PMC2947343
- DOI: 10.1038/onc.2010.63
Protein kinase Cvarepsilon mediates Stat3Ser727 phosphorylation, Stat3-regulated gene expression, and cell invasion in various human cancer cell lines through integration with MAPK cascade (RAF-1, MEK1/2, and ERK1/2)
Abstract
Protein kinase C epsilon (PKCvarepsilon), a novel calcium-independent PKC isoform, has been shown to be a transforming oncogene. PKCvarepsilon-mediated oncogenic activity is linked to its ability to promote cell survival. However, the mechanisms by which PKCvarepsilon signals cell survival remain elusive. We found that signal transducers and activators of transcription 3 (Stat3), which is constitutively activated in a wide variety of human cancers, is a protein partner of PKCvarepsilon. Stat3 has two conserved amino-acid (Tyr705 and Ser727) residues, which are phosphorylated during Stat3 activation. PKCvarepsilon interacts with Stat3alpha isoform, which has Ser727, and not with Stat3beta isoform, which lacks Ser727. PKCvarepsilon-Stat3 interaction and Stat3Ser727 phosphorylation was initially observed during induction of squamous cell carcinomas and in prostate cancer. Now we present that (1) PKCvarepsilon physically interacts with Stat3alpha isoform in various human cancer cells: skin melanomas (MeWo and WM266-4), gliomas (T98G and MO59K), bladder (RT-4 and UM-UC-3), colon (Caco-2), lung (H1650), pancreatic (PANC-1), and breast (MCF-7 and MDA:MB-231); (2) inhibition of PKCvarepsilon expression using specific siRNA inhibits Stat3Ser727 phosphorylation, Stat3-DNA binding, Stat3-regulated gene expression as well as cell invasion; and (3) PKCvarepsilon mediates Stat3Ser727 phosphorylation through integration with the MAPK cascade (RAF-1, MEK1/2, and ERK1/2). The results indicate that PKCvarepsilon-mediated Stat3Ser727 phosphorylation is essential for constitutive activation of Stat3 and cell invasion in various human cancers.
Conflict of interest statement
No conflict of Interest.
Figures
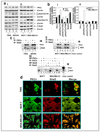



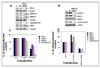
Similar articles
-
Protein kinase C epsilon, which sensitizes skin to sun's UV radiation-induced cutaneous damage and development of squamous cell carcinomas, associates with Stat3.Cancer Res. 2007 Feb 1;67(3):1385-94. doi: 10.1158/0008-5472.CAN-06-3350. Cancer Res. 2007. PMID: 17283176
-
Protein kinase Cepsilon interacts with signal transducers and activators of transcription 3 (Stat3), phosphorylates Stat3Ser727, and regulates its constitutive activation in prostate cancer.Cancer Res. 2007 Sep 15;67(18):8828-38. doi: 10.1158/0008-5472.CAN-07-1604. Cancer Res. 2007. PMID: 17875724
-
Ultraviolet radiation and 12-O-tetradecanoylphorbol-13-acetate-induced interaction of mouse epidermal protein kinase Cε with Stat3 involve integration with ERK1/2.Mol Carcinog. 2012 Apr;51(4):291-302. doi: 10.1002/mc.20776. Epub 2011 Apr 7. Mol Carcinog. 2012. PMID: 21480396 Free PMC article.
-
Protein kinase Cepsilon interacts with Stat3 and regulates its activation that is essential for the development of skin cancer.Mol Carcinog. 2007 Aug;46(8):646-53. doi: 10.1002/mc.20356. Mol Carcinog. 2007. PMID: 17583567 Review.
-
STAT3beta, a distinct isoform from STAT3.Int J Biochem Cell Biol. 2019 May;110:130-139. doi: 10.1016/j.biocel.2019.02.006. Epub 2019 Feb 26. Int J Biochem Cell Biol. 2019. PMID: 30822557 Review.
Cited by
-
Inhibition of STAT3 blocks protein synthesis and tumor metastasis in osteosarcoma cells.J Exp Clin Cancer Res. 2018 Oct 4;37(1):244. doi: 10.1186/s13046-018-0914-0. J Exp Clin Cancer Res. 2018. PMID: 30286779 Free PMC article.
-
Chk1 Inhibition Potently Blocks STAT3 Tyrosine705 Phosphorylation, DNA-Binding Activity, and Activation of Downstream Targets in Human Multiple Myeloma Cells.Mol Cancer Res. 2022 Mar 1;20(3):456-467. doi: 10.1158/1541-7786.MCR-21-0366. Mol Cancer Res. 2022. PMID: 34782371 Free PMC article.
-
Therapeutic Potential of Thymoquinone in Glioblastoma Treatment: Targeting Major Gliomagenesis Signaling Pathways.Biomed Res Int. 2018 Jan 31;2018:4010629. doi: 10.1155/2018/4010629. eCollection 2018. Biomed Res Int. 2018. PMID: 29651429 Free PMC article. Review.
-
STAT3 serine 727 phosphorylation influences clinical outcome in glioblastoma.Int J Clin Exp Pathol. 2014 May 15;7(6):3141-9. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25031733 Free PMC article.
-
Constitutive STAT3 Phosphorylation in Circulating CD4+ T Lymphocytes Associates with Disease Activity and Treatment Response in Recent-Onset Rheumatoid Arthritis.PLoS One. 2015 Sep 9;10(9):e0137385. doi: 10.1371/journal.pone.0137385. eCollection 2015. PLoS One. 2015. PMID: 26353115 Free PMC article.
References
-
- Akira S. Roles of STAT3 defined by tissue-specific gene targeting. Oncogene. 2000;19:2607–2611. - PubMed
-
- Alvarez JV, Febbo PG, Ramaswamy S, Loda M, Richardson A, Frank DA. Identification of a genetic signature of activated signal transducer and activator of transcription 3 in human tumors. Cancer Res. 2005;65:5054–5062. - PubMed
-
- Aziz MH, Manoharan HT, Church DR, Dreckschmidt NE, Zhong W, Oberley TD, et al. Protein kinase Cepsilon interacts with signal transducers and activators of transcription 3 (Stat3), phosphorylates Stat3Ser727, and regulates its constitutive activation in prostate cancer. Cancer Res. 2007a;67:8828–8838. - PubMed
-
- Aziz MH, Manoharan HT, Sand JM, Verma AK. Protein kinase Cepsilon interacts with Stat3 and regulates its activation that is essential for the development of skin cancer. Mol Carcinog. 2007b;46:646–653. - PubMed
-
- Aziz MH, Manoharan HT, Verma AK. Protein kinase C epsilon, which sensitizes skin to sun's UV radiation-induced cutaneous damage and development of squamous cell carcinomas, associates with Stat3. Cancer Res. 2007c;67:1385–1394. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

