Investigation of SNARE-Mediated Membrane Fusion Mechanism Using Atomic Force Microscopy
- PMID: 20228892
- PMCID: PMC2836841
- DOI: 10.1143/JJAP.48.08JA03
Investigation of SNARE-Mediated Membrane Fusion Mechanism Using Atomic Force Microscopy
Abstract
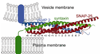
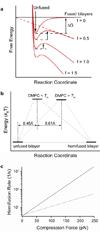
Membrane fusion is driven by specialized proteins that reduce the free energy penalty for the fusion process. In neurons and secretory cells, soluble N-ethylmaleimide-sensitive factor-attachment protein (SNAP) receptors (SNAREs) mediate vesicle fusion with the plasma membrane during vesicular content release. Although, SNAREs have been widely accepted as the minimal machinery for membrane fusion, the specific mechanism for SNARE-mediated membrane fusion remains an active area of research. Here, we summarize recent findings based on force measurements acquired in a novel experimental system that uses atomic force microscope (AFM) force spectroscopy to investigate the mechanism(s) of membrane fusion and the role of SNAREs in facilitating membrane hemifusion during SNARE-mediated fusion. In this system, protein-free and SNARE-reconstituted lipid bilayers are formed on opposite (trans) substrates and the forces required to induce membrane hemifusion and fusion or to unbind single v-/t-SNARE complexes are measured. The obtained results provide evidence for a mechanism by which the pulling force generated by interacting trans-SNAREs provides critical proximity between the membranes and destabilizes the bilayers at fusion sites by broadening the hemifusion energy barrier and consequently making the membranes more prone to fusion.
Figures








Similar articles
-
Pulling force generated by interacting SNAREs facilitates membrane hemifusion.Integr Biol (Camb). 2009 Apr;1(4):301-10. doi: 10.1039/b900685k. Epub 2009 Feb 26. Integr Biol (Camb). 2009. PMID: 20023730 Free PMC article.
-
Atomic force microscope spectroscopy reveals a hemifusion intermediate during soluble N-ethylmaleimide-sensitive factor-attachment protein receptors-mediated membrane fusion.Biophys J. 2008 Jan 15;94(2):648-55. doi: 10.1529/biophysj.107.114298. Epub 2007 Sep 14. Biophys J. 2008. PMID: 17872963 Free PMC article.
-
Close is not enough: SNARE-dependent membrane fusion requires an active mechanism that transduces force to membrane anchors.J Cell Biol. 2000 Jul 10;150(1):105-17. doi: 10.1083/jcb.150.1.105. J Cell Biol. 2000. PMID: 10893260 Free PMC article.
-
Role of SNAREs in membrane fusion.Adv Exp Med Biol. 2011;713:13-32. doi: 10.1007/978-94-007-0763-4_3. Adv Exp Med Biol. 2011. PMID: 21432012 Review.
-
TRP Channel Trafficking.In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 23. In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 23. PMID: 21204515 Free Books & Documents. Review.
Cited by
-
Unzipping of neuronal snare protein with steered molecular dynamics occurs in three steps.J Mol Model. 2014 Aug;20(8):2381. doi: 10.1007/s00894-014-2381-7. Epub 2014 Jul 31. J Mol Model. 2014. PMID: 25079079
-
Adhesion energy can regulate vesicle fusion and stabilize partially fused states.J R Soc Interface. 2012 Jul 7;9(72):1555-67. doi: 10.1098/rsif.2011.0827. Epub 2012 Jan 18. J R Soc Interface. 2012. PMID: 22258550 Free PMC article.
References
-
- Blumenthal R, Clague MJ, Durell SR, Epand RM. Chem. Rev. 2003;103:53. - PubMed
-
- Chernomordik LV, Kozlov MM. Annu. Rev. Biochem. 2003;72:175. - PubMed
-
- Fardon NJ, Wilkinson R, Thomas TH. Am. J. Hypertension. 2001;14:927. - PubMed
-
- Lentsch AB, Ward PA. J. Pathol. 2000;190:343. - PubMed
-
- Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE. Cell. 1998;92:759. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
