Effect of feeding regimens on circadian rhythms: implications for aging and longevity
- PMID: 20228939
- PMCID: PMC2837202
- DOI: 10.18632/aging.100116
Effect of feeding regimens on circadian rhythms: implications for aging and longevity
Abstract
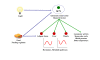
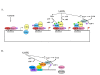
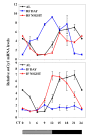
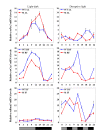
Increased longevity and improved health can be achieved in mammals by two feeding regimens, caloric restriction (CR), which limits the amount of daily calorie intake, and intermittent fasting (IF), which allows the food to be available ad libitum every other day. The precise mechanisms mediating these beneficial effects are still unresolved. Resetting the circadian clock is another intervention that can lead to increased life span and well being, while clock disruption is associated with aging and morbidity. Currently, a large body of evidence links circadian rhythms with metabolism and feeding regimens. In particular, CR, and possibly also IF, can entrain the master clock located in the suprachiasmatic nuclei (SCN) of the brain hypothalamus. These findings raise the hypothesis that the beneficial effects exerted by these feeding regimens could be mediated, at least in part, through resetting of the circadian clock, thus leading to synchrony in metabolism and physiology. This hypothesis is reinforced by a transgenic mouse model showing spontaneously reduced eating alongside robust circadian rhythms and increased life span. This review will summarize recent findings concerning the relationships between feeding regimens, circadian rhythms, and metabolism with implications for ageing attenuation and life span extension.
Keywords: aging; caloric restriction; circadian rhythms; clock; intermittent fasting; life span; metabolism; αMUPA.
Conflict of interest statement
The authors of this manuscript have no conflict of interest to declare.
Figures

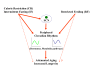
References
-
- Panda S, Hogenesch JB, Kay SA. Circadian rhythms from flies to human. Nature. 2002;417:329–335. - PubMed
-
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. - PubMed
-
- Schibler U, Ripperger J, Brown SA. Peripheral circadian oscillators in mammals: time and food. J Biol Rhythms. 2003;18:250–260. - PubMed
-
- Maron BJ, Kogan J, Proschan MA, Hecht GM, Roberts WC. Circadian variability in the occurrence of sudden cardiac death in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 1994;23:1405–1409. - PubMed
-
- Staels B. When the Clock stops ticking, metabolic syndrome explodes. Nat Med. 2006;12:54–55. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
