Cyanylated Cysteine: A Covalently Attached Vibrational Probe of Protein-Lipid Contacts
- PMID: 20228945
- PMCID: PMC2836368
- DOI: 10.1021/jz1000177
Cyanylated Cysteine: A Covalently Attached Vibrational Probe of Protein-Lipid Contacts
Abstract
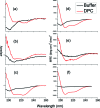
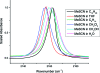
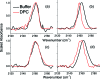
Cyanylated cysteine, or beta-thiocyanatoalanine, is an artificial amino acid that can be introduced into peptides and proteins by post-translational chemical modification of solvent-exposed cysteine side chains, and thus it can be used in any protein with a suitable expression and mutagenesis system. In this study, cyanylated cysteine is introduced at selected sites in two model peptides that have been shown to bind to membrane interfaces: a membrane-binding sequence of the human myelin basic protein and the antimicrobial peptide CM15. Far-UV circular dichroism indicates that the secondary structures of the bound peptides are not influenced by introduction of the artificial side chain. Infrared spectra of both systems in buffer and exposed to dodecylphosphocholine micelles indicate that the CN stretching absorption band of cyanylated cysteine can clearly distinguish between membrane burial and solvent exposure of the artificial side chain. Since infrared spectroscopy can be applied in a wide variety of lipid systems, and since cyanylated cysteine can be introduced into proteins of arbitrary size via mutagenesis and post-translational modification, this new probe could see wide use in characterizing the protein-lipid interactions of membrane proteins.
Figures
References
-
- Caffrey M. Crystallizing Membrane Proteins for Structure Determination: Use of Lipidic Mesophases. Annu. Rev. Biophys. 2009, 38, 29–51. - PubMed
-
- McDermott A. Structure and Dynamics of Membrane Proteins by Magic Angle Spinning Solid-State NMR. Annu. Rev. Biophys. 2009, 38, 385–403. - PubMed
- Opella S. J.; Zeri A. C.; Park S. H. Structure, Dynamics, and Assembly of Filamentous Bacteriophages by Nuclear Magnetic Resonance Spectroscopy. Annu. Rev. Phys. Chem. 2008, 59, 635–657. - PubMed
-
- Loura L. M. S.; de Almeida R. F. M.; Coutinho A.; Prieto M. Interaction of Peptides with Binary Phospholipid Membranes: Application of Fluorescence Methodologies. Chem. Phys. Lipids 2003, 122, 77–96. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

