Chronic alcohol consumption enhances myeloid-derived suppressor cells in B16BL6 melanoma-bearing mice
- PMID: 20229084
- PMCID: PMC2881944
- DOI: 10.1007/s00262-010-0837-x
Chronic alcohol consumption enhances myeloid-derived suppressor cells in B16BL6 melanoma-bearing mice
Erratum in
- Cancer Immunol Immunother. 2010 Aug;59(8):1161-2
Abstract
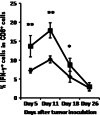
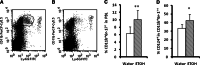
We previously found that chronic alcohol consumption decreases the survival of mice bearing subcutaneous B16BL6 melanoma. The underlying mechanism is still not completely understood. Antitumor T cell immune responses are important to inhibiting tumor progression and extending survival. Therefore, we examined the effects of chronic alcohol consumption on the functionality and regulation of these cells in C57BL/6 mice that chronically consumed 20% (w/v) alcohol and subsequently were inoculated subcutaneously with B16BL6 melanoma cells. Chronic alcohol consumption inhibited melanoma-induced memory T cell expansion and accelerated the decay of interferon (IFN)-gamma producing T cells in the tumor-bearing mice. Foxp3(+)CD4(+)CD25(+) regulatory T cells were not affected; however, the percentage of myeloid-derived suppressor cells (MDSC) was significantly increased in the peripheral blood and spleen. T cell proliferation as determined by carboxyfluorescein succinimidyl ester labeling experiments in vitro was inhibited by alcohol consumption relative to control water-drinking melanoma-bearing mice. Collectively, these data show that chronic alcohol consumption inhibits proliferation of memory T cells, accelerates the decay of IFN-gamma producing CD8(+) T cells, and increases MDSC, all of which could be associated with melanoma progression and reduced survival.
Figures




Similar articles
-
Neem leaf glycoprotein inhibits CD4+CD25+Foxp3+ Tregs to restrict murine tumor growth.Immunotherapy. 2011 Aug;3(8):949-69. doi: 10.2217/imt.11.81. Immunotherapy. 2011. PMID: 21843083
-
The immunomodulatory, antitumor and antimetastatic responses of melanoma-bearing normal and alcoholic mice to sunitinib and ALT-803: a combinatorial treatment approach.Cancer Immunol Immunother. 2016 Sep;65(9):1123-34. doi: 10.1007/s00262-016-1876-8. Epub 2016 Aug 1. Cancer Immunol Immunother. 2016. PMID: 27481107 Free PMC article.
-
Alcohol consumption and antitumor immunity: dynamic changes from activation to accelerated deterioration of the immune system.Adv Exp Med Biol. 2015;815:313-31. doi: 10.1007/978-3-319-09614-8_18. Adv Exp Med Biol. 2015. PMID: 25427915
-
IFN-γ is essential for the inhibition of B16BL6 melanoma lung metastasis in chronic alcohol drinking mice.Clin Exp Metastasis. 2011 Mar;28(3):301-7. doi: 10.1007/s10585-011-9372-1. Epub 2011 Jan 14. Clin Exp Metastasis. 2011. PMID: 21234656 Free PMC article.
-
Tumor-specific T-cell memory: clearing the regulatory T-cell hurdle.Cancer Res. 2008 Mar 15;68(6):1614-7. doi: 10.1158/0008-5472.CAN-07-6012. Cancer Res. 2008. PMID: 18339838 Free PMC article. Review.
Cited by
-
Chronic alcohol consumption decreases the percentage and number of NK cells in the peripheral lymph nodes and exacerbates B16BL6 melanoma metastasis into the draining lymph nodes.Cell Immunol. 2011;266(2):172-9. doi: 10.1016/j.cellimm.2010.10.001. Epub 2010 Oct 25. Cell Immunol. 2011. PMID: 20974468 Free PMC article.
-
Alcohol as a Non-UV Social-Environmental Risk Factor for Melanoma.Cancers (Basel). 2022 Oct 13;14(20):5010. doi: 10.3390/cancers14205010. Cancers (Basel). 2022. PMID: 36291794 Free PMC article. Review.
-
Chronic alcohol consumption inhibits peripheral NK cell development and maturation by decreasing the availability of IL-15.J Leukoc Biol. 2017 Apr;101(4):1015-1027. doi: 10.1189/jlb.1A0716-298RR. Epub 2016 Nov 11. J Leukoc Biol. 2017. PMID: 27837016 Free PMC article.
-
Skin Immunization Obviates Alcohol-Related Immune Dysfunction.Biomolecules. 2015 Nov 6;5(4):3009-28. doi: 10.3390/biom5043009. Biomolecules. 2015. PMID: 26561838 Free PMC article.
-
Chronic Alcohol Consumption Enhances Skeletal Muscle Wasting in Mice Bearing Cachectic Cancers: The Role of TNFα/Myostatin Axis.Alcohol Clin Exp Res. 2020 Jan;44(1):66-77. doi: 10.1111/acer.14221. Epub 2019 Nov 11. Alcohol Clin Exp Res. 2020. PMID: 31657476 Free PMC article.
References
-
- (2007) Food, nutrition, physical activity, and the prevention of cancer; a global perspective. World Cancer Research Fund/American Institute for Cancer Research, Washington, DC
-
- Baraona E, Zeballos GA, Shoichet L, Mak KM, Lieber CS. Ethanol consumption increases nitric oxide production in rats, and its peroxynitrite-mediated toxicity is attenuated by polyenylphosphatidylcholine. Alcohol Clin Exp Res. 2002;26:883–889. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

