Exogenous modulation of intrinsic optic nerve neuroprotective activity
- PMID: 20229104
- PMCID: PMC3678383
- DOI: 10.1007/s00417-010-1336-7
Exogenous modulation of intrinsic optic nerve neuroprotective activity
Abstract
Background: To characterize the molecular and functional status of the rat retina and optic nerve after acute elevation of intraocular pressure (IOP).
Methods: Retinal ischemia was induced in rats by increasing the IOP (110 mmHg/60 minutes). Microarray analysis, quantitative RT-PCR (qRT-PCR) and immunohistochemistry were used to characterize retinal tissue. PLGA microspheres containing neurotrophic factors (BDNF, GDNF, or CNTF) or empty microspheres were injected into the vitreous of operated animals 1 day after elevation of IOP. Pupil light reflex (PLR) parameters and electroretinograms (ERG) were monitored at multiple time points during the 60-day postoperative recovery period.
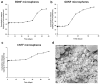
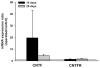
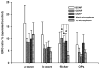
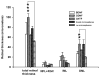
Results: Molecular analysis showed a significant intrinsic up-regulation of CNTF at 10 and 25 days after induction of the acute ocular hypertension (p = 0.0067). Molecular tissue analysis of GDNF and its receptors (GDNFR1, GDNFR2), and BDNF and its receptor (trkB) showed no change in expression. Animals that received CNTF microspheres had no significant functional recovery compared to animals which received blank microspheres (p > 0.05). Animals that received GDNF or BDNF microspheres showed significant PLR recovery (p < 0.05 and p < 0.001 respectively) compared to non-treated animals.
Conclusions: Continuous release of neurotrophic growth factors (NGFs) significantly protects optic nerve function in the experimental model of retinal ischemia observed by PLR analysis.
Figures

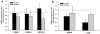
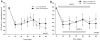

References
-
- Adachi M, Takahashi K, Nishikawa M, Miki H, Uyama M. High intraocular pressure-induced ischemia and reperfusion injury in the optic nerve and retina in rats. Graefes Arch Clin Exp Ophthalmol. 1996;234:445–451. - PubMed
-
- Hughes WF. Quantitation of ischemic damage in the rat retina. Exp Eye Res. 1991;53:573–582. - PubMed
-
- Quigley HA. Neuronal death in glaucoma. Prog Retin Eye Res. 1999;18:39–57. - PubMed
-
- Takahashi H, Goto T, Shoji T, Tanito M, Park M, Chihara E. Diabetes-associated retinal nerve fiber damage evaluated with scanning laser polarimetry. Am J Ophthalmol. 2006;142:88–94. - PubMed
-
- Ko ML, Hu DN, Ritch R, Sharma SC. The combined effect of brain-derived neurotrophic factor and a free radical scavenger in experimental glaucoma. Invest Ophthalmol Vis Sci. 2000;41:2967–2971. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

