A highly potent and selective caspase 1 inhibitor that utilizes a key 3-cyanopropanoic acid moiety
- PMID: 20229566
- PMCID: PMC3062473
- DOI: 10.1002/cmdc.200900531
A highly potent and selective caspase 1 inhibitor that utilizes a key 3-cyanopropanoic acid moiety
Abstract
Herein, we examine the potential of a nitrile-containing propionic acid moiety as an electrophile for covalent attack by the active-site cysteine residue of caspase 1. The syntheses of several cyanopropanate-containing small molecules based on the optimized peptidic scaffold of prodrug VX-765 were accomplished. These compounds were found to be potent inhibitors of caspase 1 (IC(50) values < or =1 nM). Examination of these novel small molecules against a caspase panel demonstrated an impressive degree of selectivity for caspase 1 inhibition over other caspase isozymes. Assessment of hydrolytic stability and selected ADME properties highlighted these agents as potentially useful tools for studying caspase 1 down-regulation in various settings, including in vivo analyses.
Figures
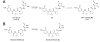
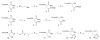

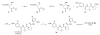
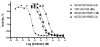
 IC50 = 11.5 nM), NCGC00185682 (3) (
IC50 = 11.5 nM), NCGC00185682 (3) ( IC50 = 144.7 nM), NCGC00183434 (4) (◆ IC50 = 0.316 nM)and the tetrazole NCGC00183681 (16) (■ IC50 = 20.4 nM).
IC50 = 144.7 nM), NCGC00183434 (4) (◆ IC50 = 0.316 nM)and the tetrazole NCGC00183681 (16) (■ IC50 = 20.4 nM).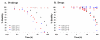

Similar articles
-
A small molecule inhibitor of Caspase 1.2010 Feb 25 [updated 2011 Mar 3]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. 2010 Feb 25 [updated 2011 Mar 3]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. PMID: 21735610 Free Books & Documents. Review.
-
(S)-1-((S)-2-{[1-(4-amino-3-chloro-phenyl)-methanoyl]-amino}-3,3-dimethyl-butanoyl)-pyrrolidine-2-carboxylic acid ((2R,3S)-2-ethoxy-5-oxo-tetrahydro-furan-3-yl)-amide (VX-765), an orally available selective interleukin (IL)-converting enzyme/caspase-1 inhibitor, exhibits potent anti-inflammatory activities by inhibiting the release of IL-1beta and IL-18.J Pharmacol Exp Ther. 2007 May;321(2):509-16. doi: 10.1124/jpet.106.111344. Epub 2007 Feb 8. J Pharmacol Exp Ther. 2007. PMID: 17289835
-
Structure-based combinatorial library design: discovery of non-peptidic inhibitors of caspases 3 and 8.J Comput Aided Mol Des. 2001 Dec;15(12):1105-17. doi: 10.1023/a:1015976725743. J Comput Aided Mol Des. 2001. PMID: 12160093
-
Conformationally constrained inhibitors of caspase-1 (interleukin-1 beta converting enzyme) and of the human CED-3 homologue caspase-3 (CPP32, apopain).Bioorg Med Chem Lett. 1998 Oct 6;8(19):2757-62. doi: 10.1016/s0960-894x(98)00498-3. Bioorg Med Chem Lett. 1998. PMID: 9873617
-
Caspase inhibitors: a pharmaceutical industry perspective.Curr Top Med Chem. 2005;5(16):1697-717. doi: 10.2174/156802605775009720. Curr Top Med Chem. 2005. PMID: 16375749 Review.
Cited by
-
Caspase Inhibition as a Possible Therapeutic Strategy for Pemphigus Vulgaris: A Systematic Review of Current Evidence.Biology (Basel). 2022 Feb 16;11(2):314. doi: 10.3390/biology11020314. Biology (Basel). 2022. PMID: 35205180 Free PMC article. Review.
-
A long way to go: caspase inhibitors in clinical use.Cell Death Dis. 2021 Oct 15;12(10):949. doi: 10.1038/s41419-021-04240-3. Cell Death Dis. 2021. PMID: 34654807 Free PMC article. Review.
-
Gasdermin D mediates host cell death but not interleukin-1β secretion in Mycobacterium tuberculosis-infected macrophages.Cell Death Discov. 2021 Oct 30;7(1):327. doi: 10.1038/s41420-021-00716-5. Cell Death Discov. 2021. PMID: 34718331 Free PMC article.
-
Exploring the ring potential of 2,4-diaminopyrimidine derivatives towards the identification of novel caspase-1 inhibitors in Alzheimer's disease therapy.J Mol Model. 2020 Mar 4;26(4):68. doi: 10.1007/s00894-020-4319-6. J Mol Model. 2020. PMID: 32130533
-
Photoactivated inhibition of cathepsin K in a 3D tumor model.Biol Chem. 2016 Jun 1;397(6):571-82. doi: 10.1515/hsz-2015-0274. Biol Chem. 2016. PMID: 26901495 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

