Nanodevices in diagnostics
- PMID: 20229595
- PMCID: PMC2962874
- DOI: 10.1002/wnan.82
Nanodevices in diagnostics
Abstract
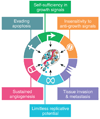
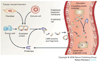
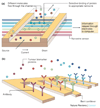
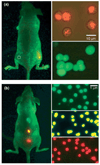
The real-time, personalized and highly sensitive early-stage diagnosis of disease remains an important challenge in modern medicine. With the ability to interact with matter at the nanoscale, the development of nanotechnology architectures and materials could potentially extend subcellular and molecular detection beyond the limits of conventional diagnostic modalities. At the very least, nanotechnology should be able to dramatically accelerate biomarker discovery, as well as facilitate disease monitoring, especially of maladies presenting a high degree of molecular and compositional heterogeneity. This article gives an overview of several of the most promising nanodevices and nanomaterials along with their applications in clinical practice. Significant work to adapt nanoscale materials and devices to clinical applications involving large interdisciplinary collaborations is already underway with the potential for nanotechnology to become an important enabling diagnostic technology.
(c) 2010 John Wiley & Sons, Inc.
Figures









References
-
- Bouck N, Stellmach V, Hsu SC. How tumors become angiogenic. Adv Cancer Res. 1996;69:135–174. - PubMed
-
- DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metabolism. 2008;7:11–20. - PubMed
-
- Cheng MMC, Cuda G, Bunimovich YL, Gaspari M, Heath JR, et al. Nanotechnologies for biomolecular detection and medical diagnostics. Curr Opin Chem Biol. 2006;10:11–19. - PubMed
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Tabár L, Fagerberg CJ, Gad A, Baldetorp L, Holmberg LH, et al. Reduction in mortality from breast cancer after mass screening with mammography. Lancet. 1985;1:829–832. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

