Caloric restriction leads to high marrow adiposity and low bone mass in growing mice
- PMID: 20229598
- PMCID: PMC3127399
- DOI: 10.1002/jbmr.82
Caloric restriction leads to high marrow adiposity and low bone mass in growing mice
Abstract
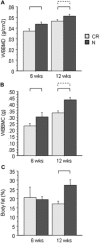
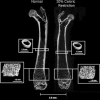
The effects of caloric restriction (CR) on the skeleton are well studied in adult rodents and include lower cortical bone mass but higher trabecular bone volume. Much less is known about how CR affects bone mass in young, rapidly growing animals. This is an important problem because low caloric intake during skeletal acquisition in humans, as in anorexia nervosa, is associated with low bone mass, increased fracture risk, and osteoporosis in adulthood. To explore this question, we tested the effect of caloric restriction on bone mass and microarchitecture during rapid skeletal growth in young mice. At 3 weeks of age, we weaned male C57Bl/6J mice onto 30% caloric restriction (10% kcal/fat) or normal diet (10% kcal/fat). Outcomes at 6 (n = 4/group) and 12 weeks of age (n = 8/group) included body mass, femur length, serum leptin and insulin-like growth factor 1 (IGF-1) values, whole-body bone mineral density (WBBMD, g/cm(2)), cortical and trabecular bone architecture at the midshaft and distal femur, bone formation and cellularity, and marrow fat measurement. Compared with the normal diet, CR mice had 52% and 88% lower serum leptin and 33% and 39% lower serum IGF-1 at 6 and 12 weeks of age (p < .05 for all). CR mice were smaller, with lower bone mineral density, trabecular, and cortical bone properties. Bone-formation indices were lower, whereas bone-resorption indices were higher (p < .01 for all) in CR versus normal diet mice. Despite having lower percent of body fat, bone marrow adiposity was elevated dramatically in CR versus normal diet mice (p < .05). Thus we conclude that caloric restriction in young, growing mice is associated with impaired skeletal acquisition, low leptin and IGF-1 levels, and high marrow adiposity. These results support the hypothesis that caloric restriction during rapid skeletal growth is deleterious to cortical and trabecular bone mass and architecture, in contrast to potential skeletal benefits of CR in aging animals.
© 2010 American Society for Bone and Mineral Research.
Figures


References
-
- Seeman E. Reduced bone density in women with fractures: contribution of low peak bone density and rapid bone loss. Osteoporos Int. 1994; 4: S15–25. - PubMed
-
- Hui SL, Slemenda CW, Johnston CC Jr. The contribution of bone loss to postmenopausal osteoporosis. Osteoporos Int. 1990; 1: 30–34. - PubMed
-
- Bonjour JP, Chevalley T, Rizzoli R, Ferrari S. Gene‐environment interactions in the skeletal response to nutrition and exercise during growth. Med Sport Sci. 2007; 51: 64–80. - PubMed
-
- Bailey DA. The Saskatchewan Pediatric Bone Mineral Accrual Study: bone mineral acquisition during the growing years. Int J Sports Med. 1997; 18: S191–194. - PubMed
-
- Bonjour JP, Theintz G, Buchs B, Slosman D, Rizzoli R. Critical years and stages of puberty for spinal and femoral bone mass accumulation during adolescence. J Clin Endocrinol Metab. 1991; 73: 555–563. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

