Uremic toxicity of indoxyl sulfate
- PMID: 20229698
- PMCID: PMC11254369
Uremic toxicity of indoxyl sulfate
Abstract
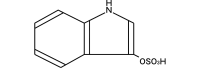
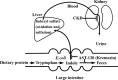
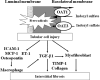
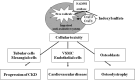
Indoxyl sulfate, a uremic toxin, is accumulated in the serum of chronic kidney disease (CKD) patients. A part of the dietary protein-derived tryptophan is metabolized into indole by tryptophanase in intestinal bacteria. Indole is absorbed into the blood from the intestine, and is metabolized to indoxyl sulfate in the liver. Indoxyl sulfate is normally excreted into urine. In CKD, however, an inadequate renal clearance of indoxyl sulfate leads to its elevated serum levels. The oral adsorbent AST-120 reduces the serum levels of indoxyl sulfate by adsorbing indole in the intestines and stimulating its excretion into feces. I have proposed a protein metabolite theory by which endogenous protein metabolites such as indoxyl sulfate play a significant role in the progression of CKD. A progressive decline in the glomerular filtration rate leads to increased serum levels of endogenous protein metabolites such as indoxyl sulfate, and to the adverse effects of their overload on the remnant nephrons. Indoxyl sulfate stimulates progressive both tubulointerstitial fibrosis and glomerular sclerosis by increasing the expression of transforming growth factor-beta1, a tissue inhibitor of metalloproteinase-1 and proalpha1 (I) collagen, leading to a further loss of nephrons. AST-120 delays the progression of CKD by removing serum indoxyl sulfate. Moreover, indoxyl sulfate induces oxidative stress in tubular cells, mesangial cells, vascular smooth muscle cells, endothelial cells and osteoblasts as well as stimulating aortic calcification in hypertensive rats, it is also involved in the progression of CKD, cardiovascular disease (CVD) and osteodystrophy. Thus, the removal of indoxyl sulfate by AST-120 ameliorates the progression of not only CKD, but also of CVD and osteodystrophy.
Figures

Similar articles
-
Indoxyl sulfate is a nephro-vascular toxin.J Ren Nutr. 2010 Sep;20(5 Suppl):S2-6. doi: 10.1053/j.jrn.2010.05.002. J Ren Nutr. 2010. PMID: 20797565 Review.
-
Role of indoxyl sulfate in the progression of chronic kidney disease and cardiovascular disease: experimental and clinical effects of oral sorbent AST-120.Ther Apher Dial. 2011 Apr;15(2):120-4. doi: 10.1111/j.1744-9987.2010.00882.x. Epub 2011 Jan 25. Ther Apher Dial. 2011. PMID: 21426500
-
The role of carbon adsorbent in the conservative management of chronic kidney disease.Panminerva Med. 2017 Jun;59(2):139-148. doi: 10.23736/S0031-0808.16.03272-9. Epub 2016 Dec 16. Panminerva Med. 2017. PMID: 27990791 Review.
-
Preventive effects of an oral sorbent on nephropathy in rats.Miner Electrolyte Metab. 1999 Jul-Dec;25(4-6):365-72. doi: 10.1159/000057476. Miner Electrolyte Metab. 1999. PMID: 10681668 Review.
-
Targeting protein-bound uremic toxins in chronic kidney disease.Expert Opin Ther Targets. 2013 Nov;17(11):1287-301. doi: 10.1517/14728222.2013.829456. Epub 2013 Aug 13. Expert Opin Ther Targets. 2013. PMID: 23941498 Review.
Cited by
-
Indoxyl Sulfate Induces Mesangial Cell Proliferation via the Induction of COX-2.Mediators Inflamm. 2016;2016:5802973. doi: 10.1155/2016/5802973. Epub 2016 Oct 23. Mediators Inflamm. 2016. PMID: 27843201 Free PMC article.
-
Paricalcitol attenuates indoxyl sulfate-induced apoptosis through the inhibition of MAPK, Akt, and NF-kB activation in HK-2 cells.Korean J Intern Med. 2019 Jan;34(1):146-155. doi: 10.3904/kjim.2016.298. Epub 2017 Oct 10. Korean J Intern Med. 2019. PMID: 28992684 Free PMC article.
-
CKD patients comorbid with hypertension are associated with imbalanced gut microbiome.iScience. 2025 Jan 9;28(2):111766. doi: 10.1016/j.isci.2025.111766. eCollection 2025 Feb 21. iScience. 2025. PMID: 39911351 Free PMC article.
-
Replacement of Dietary Carbohydrate with Protein versus Fat Differentially Alters Postprandial Circulating Hormones and Macronutrient Metabolism in Dogs.Metabolites. 2024 Jun 30;14(7):373. doi: 10.3390/metabo14070373. Metabolites. 2024. PMID: 39057696 Free PMC article.
-
Indoxyl Sulfate Activates NLRP3 Inflammasome to Induce Cardiac Contractile Dysfunction Accompanied by Myocardial Fibrosis and Hypertrophy.Cardiovasc Toxicol. 2022 Apr;22(4):365-377. doi: 10.1007/s12012-021-09718-2. Epub 2022 Jan 28. Cardiovasc Toxicol. 2022. PMID: 35088197
References
-
- Yavuz A, Tetta C, Ersoy FF, D’intini V, Ratanarat R, De Cal M, Bonello M, Bordoni V, Salvatori G, Andrikos E, Yakupoglu G, Levin NW, Ronco C. Uremic toxins: a new focus on an old subject. Semin Dial, 2005; 18: 203–211. - PubMed
-
- Vanholder R, De Smet R, Glorieux G, Argilés A, Baurmeister U, Brunet P, Clark W, Cohen G, De Deyn PP, Deppisch R, Descamps-Latscha B, Henle T, Jörres A, Lemke HD, Massy ZA, Passlick-Deetjen J, Rodriguez M, Stegmayr B, Stenvinkel P, Tetta C, Wanner C, Zidek W, European Uremic Toxin Work Group (EUTox). Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int, 2003; 63: 1934–1943. - PubMed
-
- Niwa T. Uremic Toxicity. Indoxyl sulfate. In Textbook of Nephrology, edited by Massry SG, Glassock RJ. pp. 1269–1272, 2001, Lippincott Williams & Wilkins, Philadelphia.
-
- Niwa T, Ise M. Indoxyl sulfate, a circulating uremic toxin, stimulates the progression of glomerular sclerosis. J Lab Clin Med, 1994; 124: 96–104. - PubMed
-
- Niwa T, Ise M, Miyazaki T. Progression of glomerular sclerosis in experimental uremic rats by administration of indole, a precursor of indoxyl sulfate. Am J Nephrol, 1994; 14: 207–212. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
