Crystallographic and single-crystal spectral analysis of the peroxidase ferryl intermediate
- PMID: 20230048
- PMCID: PMC3202969
- DOI: 10.1021/bi100238r
Crystallographic and single-crystal spectral analysis of the peroxidase ferryl intermediate
Abstract
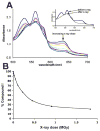
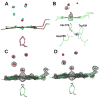
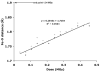
The ferryl [Fe(IV)O] intermediate is important in many heme enzymes, and thus, the precise nature of the Fe(IV)-O bond is critical in understanding enzymatic mechanisms. The 1.40 A crystal structure of cytochrome c peroxidase Compound I has been determined as a function of X-ray dose while the visible spectrum was being monitored. The Fe-O bond increases in length from 1.73 A in the low-X-ray dose structure to 1.90 A in the high-dose structure. The low-dose structure correlates well with an Fe(IV) horizontal lineO bond, while we postulate that the high-dose structure is the cryo-trapped Fe(III)-OH species previously thought to be an Fe(IV)-OH species.
Figures
Similar articles
-
Heme enzymes. Neutron cryo-crystallography captures the protonation state of ferryl heme in a peroxidase.Science. 2014 Jul 11;345(6193):193-7. doi: 10.1126/science.1254398. Epub 2014 Jul 10. Science. 2014. PMID: 25013070
-
High-resolution crystal structures and spectroscopy of native and compound I cytochrome c peroxidase.Biochemistry. 2003 May 20;42(19):5600-8. doi: 10.1021/bi034058c. Biochemistry. 2003. PMID: 12741816
-
Application of Badger's rule to heme and non-heme iron-oxygen bonds: an examination of ferryl protonation states.J Am Chem Soc. 2006 Feb 15;128(6):1902-6. doi: 10.1021/ja054074s. J Am Chem Soc. 2006. PMID: 16464091
-
On the status of ferryl protonation.J Inorg Biochem. 2006 Apr;100(4):448-59. doi: 10.1016/j.jinorgbio.2005.12.019. Epub 2006 Feb 28. J Inorg Biochem. 2006. PMID: 16500711 Review.
-
Structural analysis of compound I in hemoproteins: study on Proteus mirabilis catalase.Biochimie. 1997 Nov;79(11):667-71. doi: 10.1016/s0300-9084(97)83500-6. Biochimie. 1997. PMID: 9479449 Review.
Cited by
-
Synthetic Fe/Cu Complexes: Toward Understanding Heme-Copper Oxidase Structure and Function.Chem Rev. 2018 Nov 28;118(22):10840-11022. doi: 10.1021/acs.chemrev.8b00074. Epub 2018 Oct 29. Chem Rev. 2018. PMID: 30372042 Free PMC article. Review.
-
A nearly on-axis spectroscopic system for simultaneously measuring UV-visible absorption and X-ray diffraction in the SPring-8 structural genomics beamline.J Synchrotron Radiat. 2016 Jan;23(1):334-8. doi: 10.1107/S1600577515018275. Epub 2016 Jan 1. J Synchrotron Radiat. 2016. PMID: 26698082 Free PMC article.
-
Goniometer-based femtosecond crystallography with X-ray free electron lasers.Proc Natl Acad Sci U S A. 2014 Dec 2;111(48):17122-7. doi: 10.1073/pnas.1418733111. Epub 2014 Oct 31. Proc Natl Acad Sci U S A. 2014. PMID: 25362050 Free PMC article.
-
A Noncanonical Tryptophan Analogue Reveals an Active Site Hydrogen Bond Controlling Ferryl Reactivity in a Heme Peroxidase.JACS Au. 2021 Jul 26;1(7):913-918. doi: 10.1021/jacsau.1c00145. Epub 2021 May 14. JACS Au. 2021. PMID: 34337604 Free PMC article.
-
Macromolecular crystallography and biology at the Linac Coherent Light Source.J Synchrotron Radiat. 2025 May 1;32(Pt 3):548-566. doi: 10.1107/S1600577525002735. Epub 2025 Apr 23. J Synchrotron Radiat. 2025. PMID: 40266725 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

