Sequential rearrangement of interhelical networks upon rhodopsin activation in membranes: the Meta II(a) conformational substate
- PMID: 20230054
- PMCID: PMC2859452
- DOI: 10.1021/ja910317a
Sequential rearrangement of interhelical networks upon rhodopsin activation in membranes: the Meta II(a) conformational substate
Abstract
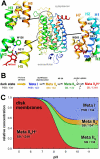
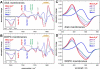
Photon absorption by rhodopsin is proposed to lead to an activation pathway that is described by the extended reaction scheme Meta I <==>Meta II(a) <==> Meta II(b) <==> Meta II(b)H(+), where Meta II(b)H(+) is thought to be the conformational substate that activates the G protein transducin. Here we test this extended scheme for rhodopsin in a membrane bilayer environment by investigating lipid perturbation of the activation mechanism. We found that symmetric membrane lipids having two unsaturated acyl chains, such as 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), selectively stabilize the Meta II(a) substate in the above mechanism. By combining FTIR and UV-visible difference spectroscopy, we characterized the structural and functional changes involved in the transition to the Meta II(a) intermediate, which links the inactive Meta I intermediate with the Meta II(b) states formed by helix rearrangement. Besides the opening of the Schiff base ionic lock, the Meta II(a) substate is characterized by an activation switch in a conserved water-mediated hydrogen-bonded network involving transmembrane helices H1/H2/H7, which is sensed by its key residue Asp83. On the other hand, movement of retinal toward H5 and its interaction with another interhelical H3/H5 network mediated by His211 and Glu122 is absent in Meta II(a). The latter rearrangement takes place only in the subsequent transition to Meta II(b), which has been previously associated with movement of H6. Our results imply that activating structural changes in the H1/H2/H7 network are triggered by disruption of the Schiff base salt bridge and occur prior to other chromophore-induced changes in the H3/H5 network and the outward tilt of H6 in the activation process.
Figures

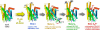

References
-
- Hofmann KP, Scheerer P, Hildebrand PW, Choe HW, Park JH, Heck M, Ernst OP. Trends Biochem. Sci. 2009;34:540–552. - PubMed
-
- Ahuja S, Smith SO. Trends Pharmacol. Sci. 2009;30:494–502. - PubMed
-
- Oldham WM, Hamm HE. Nat. Rev. Mol. Cell Biol. 2008;9:60–71. - PubMed
-
- Lewis JW, Kliger DS. Methods Enzymol. 2000;315:164–178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

