Cancer-derived mutations in the fibronectin III repeats of PTPRT/PTPrho inhibit cell-cell aggregation
- PMID: 20230342
- PMCID: PMC2921943
- DOI: 10.3109/15419061003653771
Cancer-derived mutations in the fibronectin III repeats of PTPRT/PTPrho inhibit cell-cell aggregation
Abstract
Abstract The receptor protein tyrosine phosphatase T PTPrho is the most frequently mutated tyrosine phosphatase in human cancer. PTPrho mediates homophilic cell-cell aggregation. In its extracellular region, PTPrho has cell adhesion molecule-like motifs, including a MAM domain, an immunoglobulin domain, and four fibronectin type III (FNIII) repeats. Tumor-derived mutations have been identified in all of these extracellular domains. Previously, the authors determined that tumor-derived mutations in the MAM and immunoglobulin domains of PTPrho reduce homophilic cell-cell aggregation. In this paper, the authors describe experiments in which the contribution of the FNIII repeats to PTPrho-mediated cell-cell adhesion was evaluated. The results demonstrate that deletion of the FNIII repeats of PTPrho result in defective cell-cell aggregation. Furthermore, all of the tumor-derived mutations in the FNIII repeats of PTPrho also disrupt cell-cell aggregation. These results further support the hypothesis that mutational inactivation of PTPrho may lead to cancer progression by disrupting cell-cell adhesion.
Figures
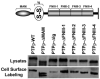

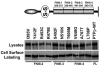

Similar articles
-
Tumor-derived extracellular mutations of PTPRT /PTPrho are defective in cell adhesion.Mol Cancer Res. 2008 Jul;6(7):1106-13. doi: 10.1158/1541-7786.MCR-07-2123. Mol Cancer Res. 2008. PMID: 18644975 Free PMC article.
-
Characterization of the adhesive properties of the type IIb subfamily receptor protein tyrosine phosphatases.Cell Commun Adhes. 2010 Apr;17(2):34-47. doi: 10.3109/15419061.2010.487957. Cell Commun Adhes. 2010. PMID: 20521994 Free PMC article.
-
Homophilic adhesion mediated by the neural cell adhesion molecule involves multiple immunoglobulin domains.Proc Natl Acad Sci U S A. 1996 Apr 30;93(9):4071-5. doi: 10.1073/pnas.93.9.4071. Proc Natl Acad Sci U S A. 1996. PMID: 8633018 Free PMC article.
-
Receptor protein tyrosine phosphatase micro: measuring where to stick.Biochem Soc Trans. 2008 Apr;36(Pt 2):167-72. doi: 10.1042/BST0360167. Biochem Soc Trans. 2008. PMID: 18363557 Review.
-
Regulation of development and cancer by the R2B subfamily of RPTPs and the implications of proteolysis.Semin Cell Dev Biol. 2015 Jan;37:108-18. doi: 10.1016/j.semcdb.2014.09.004. Epub 2014 Sep 16. Semin Cell Dev Biol. 2015. PMID: 25223585 Free PMC article. Review.
Cited by
-
Artificial Intelligence-Based Computational Screening and Functional Assays Identify Candidate Small Molecule Antagonists of PTPmu-Dependent Adhesion.Int J Mol Sci. 2023 Feb 21;24(5):4274. doi: 10.3390/ijms24054274. Int J Mol Sci. 2023. PMID: 36901713 Free PMC article.
-
Functional genetic variants in the 3'UTR of PTPRD associated with the risk of gestational diabetes mellitus.Exp Ther Med. 2021 Jun;21(6):562. doi: 10.3892/etm.2021.9994. Epub 2021 Mar 26. Exp Ther Med. 2021. PMID: 33850534 Free PMC article.
-
PTPRT Could Be a Treatment Predictive and Prognostic Biomarker for Breast Cancer.Biomed Res Int. 2021 Aug 10;2021:3301402. doi: 10.1155/2021/3301402. eCollection 2021. Biomed Res Int. 2021. PMID: 34414233 Free PMC article.
-
Cross-talk between phospho-STAT3 and PLCγ1 plays a critical role in colorectal tumorigenesis.Mol Cancer Res. 2011 Oct;9(10):1418-28. doi: 10.1158/1541-7786.MCR-11-0147. Epub 2011 Aug 12. Mol Cancer Res. 2011. PMID: 21840932 Free PMC article.
-
Receptor protein tyrosine phosphatases and cancer: new insights from structural biology.Cell Adh Migr. 2012 Jul-Aug;6(4):356-64. doi: 10.4161/cam.21242. Epub 2012 Jul 1. Cell Adh Migr. 2012. PMID: 22796942 Free PMC article. Review.
References
-
- Aricescu AR, Siebold C, Choudhuri K, Chang VT, Lu W, Davis SJ, van der Merwe PA, Jones EY. Structure of a tyrosine phosphatase adhesive interaction reveals a spacer-clamp mechanism. Science. 2007;317:1217–20. - PubMed
-
- Aricescu AR, Siebold C, Jones EY. Receptor protein tyrosine phosphatase mu: measuring where to stick. Biochem Soc Trans. 2008;36:167–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
