Transient receptor potential channels in pain and inflammation: therapeutic opportunities
- PMID: 20230457
- PMCID: PMC3112370
- DOI: 10.1111/j.1533-2500.2010.00358.x
Transient receptor potential channels in pain and inflammation: therapeutic opportunities
Abstract
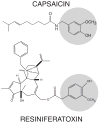
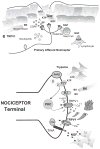
In ancient times, physicians had a limited number of therapies to provide pain relief. Not surprisingly, plant extracts applied topically often served as the primary analgesic plan. With the discovery of the capsaicin receptor (transient receptor potential cation channel, subfamily V, member 1 [TRPV1]), the search for "new" analgesics has returned to compounds used by physicians thousands of years ago. One such compound, capsaicin, couples the paradoxical action of nociceptor activation (burning pain) with subsequent analgesia following repeat or high-dose application. Investigating this "paradoxical" action of capsaicin has revealed several overlapping and complementary mechanisms to achieve analgesia including receptor desensitization, nociceptor dysfunction, neuropeptide depletion, and nerve terminal destruction. Moreover, the realization that TRPV1 is both sensitized and activated by endogenous products of inflammation, including bradykinin, H+, adenosine triphosphate, fatty acid derivatives, nerve growth factor, and trypsins, has renewed interest in TRPV1 as an important site of analgesia. Building on this foundation, a new series of preclinical and clinical studies targeting TRPV1 has been reported. These include trials using brief exposure to high-dose topical capsaicin in conjunction with prior application of a local anesthetic. Clinical use of resiniferatoxin, another ancient but potent TRPV1 agonist, is also being explored as a therapy for refractory pain. The development of orally administered high-affinity TRPV1 antagonists holds promise for pioneering a new generation of analgesics capable of blocking painful sensations at the site of inflammation and tissue injury. With the isolation of other members of the TRP channel family such as TRP cation channel, subfamily A, member 1, additional opportunities are emerging in the development of safe and effective analgesics.
Figures
Similar articles
-
Prolonged analgesic response of cornea to topical resiniferatoxin, a potent TRPV1 agonist.Pain. 2010 Jun;149(3):522-528. doi: 10.1016/j.pain.2010.03.024. Epub 2010 Apr 18. Pain. 2010. PMID: 20403666 Free PMC article.
-
TRPs and pain.Semin Immunopathol. 2016 May;38(3):277-91. doi: 10.1007/s00281-015-0526-0. Epub 2015 Sep 15. Semin Immunopathol. 2016. PMID: 26374740 Review.
-
Targeting TRP channels for pain relief.Eur J Pharmacol. 2013 Sep 15;716(1-3):61-76. doi: 10.1016/j.ejphar.2013.03.003. Epub 2013 Mar 14. Eur J Pharmacol. 2013. PMID: 23500195 Review.
-
Capsaicin (TRPV1 Agonist) therapy for pain relief: farewell or revival?Clin J Pain. 2008 Feb;24(2):142-54. doi: 10.1097/AJP.0b013e318158ed9e. Clin J Pain. 2008. PMID: 18209521 Review.
-
Transient receptor potential ion channels as targets for the discovery of pain therapeutics.Curr Opin Investig Drugs. 2005 Jan;6(1):48-57. Curr Opin Investig Drugs. 2005. PMID: 15675603 Review.
Cited by
-
Mechanism for Regulation of Melanoma Cell Death via Activation of Thermo-TRPV4 and TRPV2.J Oncol. 2019 Feb 7;2019:7362875. doi: 10.1155/2019/7362875. eCollection 2019. J Oncol. 2019. PMID: 30881453 Free PMC article.
-
N-Arachidonoyl Dopamine Modulates Acute Systemic Inflammation via Nonhematopoietic TRPV1.J Immunol. 2017 Aug 15;199(4):1465-1475. doi: 10.4049/jimmunol.1602151. Epub 2017 Jul 12. J Immunol. 2017. PMID: 28701511 Free PMC article.
-
In Vitro Cytotoxic Protective Effect of Alginate-Encapsulated Capsaicin Might Improve Skin Side Effects Associated with the Topical Application of Capsaicin.Molecules. 2021 Mar 7;26(5):1455. doi: 10.3390/molecules26051455. Molecules. 2021. PMID: 33800110 Free PMC article.
-
Mechanisms of pain in sickle cell disease.Br J Pain. 2021 May;15(2):213-220. doi: 10.1177/2049463720920682. Epub 2020 May 22. Br J Pain. 2021. PMID: 34055342 Free PMC article.
-
Thermosensory Roles of G Protein-Coupled Receptors and Other Cellular Factors in Animals.Bioessays. 2025 Mar;47(3):e202400233. doi: 10.1002/bies.202400233. Epub 2024 Dec 26. Bioessays. 2025. PMID: 39723698 Free PMC article. Review.
References
-
- Appendino G, Szallasi A. Euphorbium: modern research on its active principle, resiniferatoxin, revives an ancient medicine. Life Sci. 1997;60:681–696. - PubMed
-
- Fields HL. Pain syndromes in neurology. London, Boston: Butterworths-Heinemann Ltd; 1990.
-
- Carpenter MB. Core text of neuroanatomy. 3. Baltimore: Williams & Wilkins; 1985.
-
- Cervero F. Sensory innervation of the viscera: peripheral basis of visceral pain. Physiol Rev. 1994;74:95–138. - PubMed
-
- Janig W, Khasar SG, Levine JD, Miao FJ. The role of vagal visceral afferents in the control of nociception. Prog Brain Res. 2000;122:273–287. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

