Effectiveness of adalimumab in treating patients with ankylosing spondylitis associated with enthesitis and peripheral arthritis
- PMID: 20230622
- PMCID: PMC2888191
- DOI: 10.1186/ar2953
Effectiveness of adalimumab in treating patients with ankylosing spondylitis associated with enthesitis and peripheral arthritis
Abstract
Introduction: The purpose of this study was to investigate the effectiveness of adalimumab in enthesitis and peripheral arthritis in patients with ankylosing spondylitis (AS).
Methods: Adults with active AS (Bath ankylosing spondylitis disease activity index [BASDAI] > or = 4) received adalimumab 40 mg every other week with standard antirheumatic therapies in a 12-week, open-label study. Effectiveness in enthesitis was assessed using the Maastricht ankylosing spondylitis enthesitis score (MASES, 0-13) and by examining the plantar fascia in patients with enthesitis (> or = 1 inflamed enthesis) at baseline; effectiveness in peripheral arthritis was evaluated using tender and swollen joint counts (TJC, 0-46; SJC, 0-44) in patients with peripheral arthritis (> or = 1 swollen joint) at baseline. Overall effectiveness measures included Assessment of SpondyloArthritis International Society 20% response (ASAS20).
Results: Of 1,250 patients enrolled, 686 had enthesitis and 281 had peripheral arthritis. In 667 patients with MASES > or = 1 at baseline, the median MASES was reduced from 5 at baseline to 1 at week 12. At week 12, inflammation of the plantar fascia ceased in 122 of 173 patients with inflammation at baseline. The median TJC in 281 patients with SJC > or = 1 at baseline was reduced from 5 at baseline to 1 at week 12; the median SJC improved from 2 to 0. ASAS20 responses were achieved by 70.5% of 457 patients with no enthesitis and no arthritis; 71.0% of 512 patients with only enthesitis; 68.0% of 107 patients with only arthritis; and 66.7% of 174 patients with both.
Conclusions: Treatment with adalimumab improved enthesitis and peripheral arthritis in patients with active AS.
Trial registration: ClinicalTrials.gov NCT00478660.
Figures


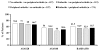
Similar articles
-
Frequency of peripheral diseases in Korean patients with ankylosing spondylitis and the effectiveness of adalimumab.Int J Rheum Dis. 2020 Aug;23(9):1175-1183. doi: 10.1111/1756-185X.13917. Epub 2020 Jul 29. Int J Rheum Dis. 2020. PMID: 32725789 Free PMC article.
-
Clinical manifestations and responsiveness to adalimumab are similar in patients with ankylosing spondylitis with and without concomitant psoriasis.Rheumatology (Oxford). 2010 Aug;49(8):1578-89. doi: 10.1093/rheumatology/keq129. Epub 2010 May 6. Rheumatology (Oxford). 2010. PMID: 20448079 Clinical Trial.
-
Effectiveness and safety of adalimumab in patients with ankylosing spondylitis or psoriatic arthritis and history of anti-tumor necrosis factor therapy.Arthritis Res Ther. 2010;12(3):R117. doi: 10.1186/ar3054. Epub 2010 Jun 16. Arthritis Res Ther. 2010. PMID: 20553600 Free PMC article. Clinical Trial.
-
[Autoimmune aspects of treatment with TNF-alpha inhibitors].Postepy Hig Med Dosw (Online). 2007 Aug 28;61:478-84. Postepy Hig Med Dosw (Online). 2007. PMID: 17786135 Review. Polish.
-
Efficacy and safety of adalimumab in ankylosing spondylitis.Open Access Rheumatol. 2014 Aug 13;6:83-90. doi: 10.2147/OARRR.S44550. eCollection 2014. Open Access Rheumatol. 2014. PMID: 27790037 Free PMC article. Review.
Cited by
-
Enthesitis indices identify different patients with this characteristic in axial and peripheral spondyloarthritis and also in psoriatic arthritis: ASAS-PerSpA data.Arthritis Res Ther. 2023 Jun 8;25(1):99. doi: 10.1186/s13075-023-03080-0. Arthritis Res Ther. 2023. PMID: 37291655 Free PMC article.
-
Characterization of Patients With Axial Spondyloarthritis by Enthesitis Presence: Data from the Corrona Psoriatic Arthritis/Spondyloarthritis Registry.ACR Open Rheumatol. 2020 Jul;2(7):449-456. doi: 10.1002/acr2.11154. Epub 2020 Jul 6. ACR Open Rheumatol. 2020. PMID: 32627974 Free PMC article.
-
Entheseal involvement in systemic disorders.Clin Rheumatol. 2015 Dec;34(12):2001-10. doi: 10.1007/s10067-015-3068-x. Epub 2015 Sep 10. Clin Rheumatol. 2015. PMID: 26354427 Review.
-
Prevalence and progression of radiographic enthesopathy at hip and pelvis in patients with axial spondyloarthritis based on CT assessment.Clin Rheumatol. 2025 Jul;44(7):2809-2818. doi: 10.1007/s10067-025-07535-4. Epub 2025 Jun 14. Clin Rheumatol. 2025. PMID: 40516015
-
Developments in therapies for spondyloarthritis.Nat Rev Rheumatol. 2012 Apr 10;8(5):280-7. doi: 10.1038/nrrheum.2012.40. Nat Rev Rheumatol. 2012. PMID: 22487798 Review.
References
-
- Rudwaleit M, Claudepierre P, Wordsworth P, Cortina EL, Sieper J, Kron M, Carcereri-De-Prati R, Kupper H, Kary S. Effectiveness, safety, and predictors of good clinical response in 1250 patients treated with adalimumab for active ankylosing spondylitis. J Rheumatol. 2009;36:801–808. doi: 10.3899/jrheum.081048. - DOI - PubMed
-
- Cruyssen B Vander, Ribbens C, Boonen A, Mielants H, de Vlam K, Lenaerts J, Steinfeld S, Bosch F Van den, Dewulf L, Vastesaeger N. The epidemiology of ankylosing spondylitis and the commencement of anti-TNF therapy in daily rheumatology practice. Ann Rheum Dis. 2007;66:1072–1077. doi: 10.1136/ard.2006.064543. - DOI - PMC - PubMed
-
- Collantes E, Zarco P, Muñoz E, Juanola X, Mulero J, Fernández-Sueiro JL, Torre-Alonso JC, Gratacós J, González C, Batlle E, Fernández P, Linares LF, Brito E, Carmona L. Disease pattern of spondyloarthropathies in Spain: description of the first national registry (REGISPONSER) extended report. Rheumatology. 2007;46:1309–1315. doi: 10.1093/rheumatology/kem084. - DOI - PubMed
-
- Brophy S, Calin A. Ankylosing spondylitis: interaction between genes, joints, age at onset, and disease expression. J Rheumatol. 2001;28:2283–2288. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

