How do hypertrophic cardiomyopathy mutations affect myocardial function in carriers with normal wall thickness? Assessment with cardiovascular magnetic resonance
- PMID: 20230637
- PMCID: PMC2842263
- DOI: 10.1186/1532-429X-12-13
How do hypertrophic cardiomyopathy mutations affect myocardial function in carriers with normal wall thickness? Assessment with cardiovascular magnetic resonance
Abstract
Background: Clinical data on myocardial function in HCM mutation carriers (carriers) is sparse but suggests that subtle functional abnormalities can be measured with tissue Doppler imaging before the development of overt hypertrophy. We aimed to confirm the presence of functional abnormalities using cardiovascular magnetic resonance (CMR), and to investigate if sensitive functional assessment could be employed to identify carriers.
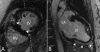
Results: 28 carriers and 28 controls were studied. Global left atrial (LA) and left ventricular (LV) dimensions, segmental peak systolic circumferential strain (SCS) and peak diastolic circumferential strain rate (DCSR), as well as the presence of late Gadolinium enhancement (LGE) were determined with CMR. Septal and lateral myocardial velocities were measured with echocardiographic tissue Doppler imaging. LV mass and volumes were comparable between groups. Maximal septal to lateral wall thickness ratio (SL ratio) was larger in carriers than in controls (1.3+/-0.2 versus 1.1+/-0.1, p<0.001). Also, LA volumes were larger in carriers compared to controls (p<0.05). Both peak SCS (p<0.05) and peak DCSR (p<0.01) were lower in carriers compared to controls, particularly in the basal lateral wall. Focal LGE was present in 2 carriers and not in controls. The combination of a SL ratio>1.2 and a peak DCSR<105%.s-1 was present in 45% of carriers and in none of the controls, yielding a positive predictive value of 100%. Two carriers and 18 controls had a SL ratio<1.2 and peak DCSR>105%.s-1, yielding a negative predictive value of 90%. With multivariate analysis, HCM mutation carriership was an independent determinant of reduced peak SCS and peak DCSR.
Conclusions: HCM mutation carriership is an independent determinant of reduced peak SCS and peak DCSR when LV wall thickness is within normal limits, and is associated with increased LA volumes and SL ratio. Using SL ratio and peak DCSR has a high accuracy to identify carriers. However, since carriers also display structural abnormalities and focal LGE, we advocate to also evaluate morphology and presence of LGE when screening for carriers.
Figures

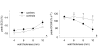

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

