Temperature-dependent phase behavior and protein partitioning in giant plasma membrane vesicles
- PMID: 20230780
- PMCID: PMC2908281
- DOI: 10.1016/j.bbamem.2010.03.009
Temperature-dependent phase behavior and protein partitioning in giant plasma membrane vesicles
Abstract
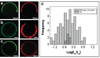
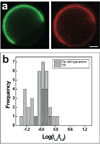
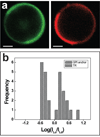
Liquid-ordered (Lo) and liquid-disordered (Ld) phase coexistence has been suggested to partition the plasma membrane of biological cells into lateral compartments, allowing for enrichment or depletion of functionally relevant molecules. This dynamic partitioning might be involved in fine-tuning cellular signaling fidelity through coupling to the plasma membrane protein and lipid composition. In earlier work, giant plasma membrane vesicles, obtained by chemically induced blebbing from cultured cells, were observed to reversibly phase segregate at temperatures significantly below 37 degrees C. In this contribution, we compare the temperature dependence of fluid phase segregation in HeLa and rat basophilic leukemia (RBL) cells. We find an essentially monotonic temperature dependence of the number of phase-separated vesicles in both cell types. We also observe a strikingly broad distribution of phase transition temperatures in both cell types. The binding of peripheral proteins, such as cholera toxin subunit B (CTB), as well as Annexin V, is observed to modulate phase transition temperatures, indicating that peripheral protein binding may be a regulator for lateral heterogeneity in vivo. The partitioning of numerous signal protein anchors and full length proteins is investigated. We find Lo phase partitioning for several proteins assumed in the literature to be membrane raft associated, but observe deviations from this expectation for other proteins, including caveolin-1.
Copyright 2010 Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Partitioning, diffusion, and ligand binding of raft lipid analogs in model and cellular plasma membranes.Biochim Biophys Acta. 2012 Jul;1818(7):1777-84. doi: 10.1016/j.bbamem.2012.03.007. Biochim Biophys Acta. 2012. PMID: 22450237
-
Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles.Proc Natl Acad Sci U S A. 2007 Feb 27;104(9):3165-70. doi: 10.1073/pnas.0611357104. Epub 2007 Feb 21. Proc Natl Acad Sci U S A. 2007. PMID: 17360623 Free PMC article.
-
Curvature- and Phase-Induced Protein Sorting Quantified in Transfected Cell-Derived Giant Vesicles.ACS Nano. 2019 Jun 25;13(6):6689-6701. doi: 10.1021/acsnano.9b01052. Epub 2019 Jun 14. ACS Nano. 2019. PMID: 31199124
-
Partitioning of membrane molecules between raft and non-raft domains: insights from model-membrane studies.Biochim Biophys Acta. 2005 Dec 30;1746(3):193-202. doi: 10.1016/j.bbamcr.2005.09.003. Epub 2005 Sep 23. Biochim Biophys Acta. 2005. PMID: 16271405 Review.
-
The mystery of membrane organization: composition, regulation and roles of lipid rafts.Nat Rev Mol Cell Biol. 2017 Jun;18(6):361-374. doi: 10.1038/nrm.2017.16. Epub 2017 Mar 30. Nat Rev Mol Cell Biol. 2017. PMID: 28356571 Free PMC article. Review.
Cited by
-
Determination of the Membrane Environment of CD59 in Living Cells.Biomolecules. 2018 May 17;8(2):28. doi: 10.3390/biom8020028. Biomolecules. 2018. PMID: 29772810 Free PMC article.
-
In situ quantification of protein binding to the plasma membrane.Biophys J. 2015 Jun 2;108(11):2648-57. doi: 10.1016/j.bpj.2015.04.021. Biophys J. 2015. PMID: 26039166 Free PMC article.
-
Association of influenza virus proteins with membrane rafts.Adv Virol. 2011;2011:370606. doi: 10.1155/2011/370606. Epub 2011 Jul 25. Adv Virol. 2011. PMID: 22312341 Free PMC article.
-
A novel biotinylated lipid raft reporter for electron microscopic imaging of plasma membrane microdomains.J Lipid Res. 2012 Oct;53(10):2214-2225. doi: 10.1194/jlr.D026468. Epub 2012 Jul 20. J Lipid Res. 2012. PMID: 22822037 Free PMC article.
-
Bile acids modulate signaling by functional perturbation of plasma membrane domains.J Biol Chem. 2013 Dec 13;288(50):35660-70. doi: 10.1074/jbc.M113.519116. Epub 2013 Oct 28. J Biol Chem. 2013. PMID: 24165125 Free PMC article.
References
-
- Simons K, Ikonen E. Functional Rafts in Cell Membranes. Nature. 1997;387:569–572. - PubMed
-
- Lichtenberg D, Goni FM, Heerklotz H. Detergent-resistant membranes should not be identified with membrane rafts. Trends in Biochemical Sciences. 2005;30:430–436. - PubMed
-
- Veatch SL, Cicuta P, Sengupta P, Honerkamp-Smith A, Holowka D, Baird B. Critical fluctuations in plasma membrane vesicles. Acs Chemical Biology. 2008;3:287–293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

