A novel in vivo regulatory role of P-glycoprotein in alloimmunity
- PMID: 20230790
- PMCID: PMC3756468
- DOI: 10.1016/j.bbrc.2010.03.040
A novel in vivo regulatory role of P-glycoprotein in alloimmunity
Abstract
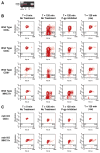
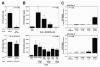
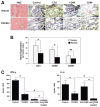
P-glycoprotein (P-gp) is required for adaptive immunity through defined functions in T cell activation and antigen presenting cell (APC) maturation. The potential role of P-gp as an in vivo regulator of alloimmunity is currently unknown. Here we show that P-gp blockade prolongs graft survival in a murine heterotopic cardiac allotransplantation model through in vivo inhibition of the T helper 1 (Th1) cytokine IFN-gamma and the Th2 product IL-4, and via downregulation of the APC-expressed positive costimulatory molecule CD80. In vitro, the P-gp antagonist PSC833, a non-calcineurin-inhibitory cyclosporine A analogue, specifically inhibited cellular efflux of the P-gp substrate rhodamine-123 in wild-type CD3(+) T cells and MHC class II(+) APCs but not their P-gp knockout counterparts that lacked rhodamine-123 efflux capacity. Additionally, P-gp blockade significantly inhibited murine alloimmune T cell activation in a dose-dependent fashion. In vivo, P-gp blockade significantly prolonged graft survival in Balb/c recipients of C57BL/6 cardiac allografts from 8.5+/-0.5 to 11.7+/-0.5 days (P<0.01), similar in magnitude to the effects of monotherapy with cyclosporine A. Moreover, P-gp blockade, compared to controls, attenuated intragraft expression of CD3 and CD80, but not CD86, and inhibited IFN-gamma and IL-4 production (P<0.05). In the setting of systemic CD86 inhibition, P-gp blockade suppressed IFN-gamma and IL-4 production significantly further (to 98% and 89% inhibition, respectively) compared to either P-gp or anti-CD86 blockade alone, and markedly prolonged allograft survival compared to anti-CD86 blockade alone (40.5+/-4.6 versus 22.5+/-2.6 days, respectively, P<0.01). Our findings define a novel in vivo regulatory role of P-gp in alloimmunity and identify P-gp as a potential therapeutic target in allotransplantation.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
P-glycoprotein functions as a differentiation switch in antigen presenting cell maturation.Am J Transplant. 2006 Dec;6(12):2884-93. doi: 10.1111/j.1600-6143.2006.01561.x. Am J Transplant. 2006. PMID: 17083370
-
Costimulatory function and expression of CD40 ligand, CD80, and CD86 in vascularized murine cardiac allograft rejection.Proc Natl Acad Sci U S A. 1996 Nov 26;93(24):13967-72. doi: 10.1073/pnas.93.24.13967. Proc Natl Acad Sci U S A. 1996. PMID: 8943044 Free PMC article.
-
Specific MDR1 P-glycoprotein blockade inhibits human alloimmune T cell activation in vitro.J Immunol. 2001 Feb 15;166(4):2451-9. doi: 10.4049/jimmunol.166.4.2451. J Immunol. 2001. PMID: 11160305
-
T-cell alterations in cardiac allograft recipients after B7 (CD80 and CD86) blockade.Transplantation. 1998 Jul 15;66(1):14-20. doi: 10.1097/00007890-199807150-00003. Transplantation. 1998. PMID: 9679816
-
P-glycoprotein--a novel therapeutic target for immunomodulation in clinical transplantation and autoimmunity?Curr Drug Targets. 2003 Aug;4(6):469-76. doi: 10.2174/1389450033490894. Curr Drug Targets. 2003. PMID: 12866661 Review.
Cited by
-
Isolation of tumorigenic circulating melanoma cells.Biochem Biophys Res Commun. 2010 Nov 26;402(4):711-7. doi: 10.1016/j.bbrc.2010.10.091. Epub 2010 Oct 25. Biochem Biophys Res Commun. 2010. PMID: 20977885 Free PMC article.
-
Dendritic cells phenotype fitting under hypoxia or lipopolysaccharide; adenosine 5'-triphosphate-binding cassette transporters far beyond an efflux pump.Clin Exp Immunol. 2013 Jun;172(3):444-54. doi: 10.1111/cei.12067. Clin Exp Immunol. 2013. PMID: 23600833 Free PMC article.
-
ABCB5 Identifies Immunoregulatory Dermal Cells.Cell Rep. 2015 Sep 8;12(10):1564-74. doi: 10.1016/j.celrep.2015.08.010. Epub 2015 Aug 28. Cell Rep. 2015. PMID: 26321644 Free PMC article.
-
Genetically determined ABCB5 functionality correlates with pigmentation phenotype and melanoma risk.Biochem Biophys Res Commun. 2013 Jul 5;436(3):536-42. doi: 10.1016/j.bbrc.2013.06.006. Epub 2013 Jun 12. Biochem Biophys Res Commun. 2013. PMID: 23770371 Free PMC article.
-
P-Glycoprotein as a Therapeutic Target in Hematological Malignancies: A Challenge to Overcome.Int J Mol Sci. 2025 May 14;26(10):4701. doi: 10.3390/ijms26104701. Int J Mol Sci. 2025. PMID: 40429842 Free PMC article. Review.
References
-
- Drach D, Zhao S, Drach J, Mahadevia R, Gattringer C, Huber H, Andreeff M. Subpopulations of normal peripheral blood and bone marrow cells express a functional multidrug resistant phenotype. Blood. 1992;80:2729–34. - PubMed
-
- Klimecki WT, Futscher BW, Grogan TM, Dalton WS. P-glycoprotein expression and function in circulating blood cells from normal volunteers. Blood. 1994;83:2451–8. - PubMed
-
- Frank MH, Denton MD, Alexander SI, Khoury SJ, Sayegh MH, Briscoe DM. Specific MDR1 P-glycoprotein blockade inhibits human alloimmune T cell activation in vitro. J Immunol. 2001;166:2451–9. - PubMed
-
- Gupta S, Kim CH, Tsuruo T, Gollapudi S. Preferential expression and activity of multidrug resistance gene 1 product (P-glycoprotein), a functionally active efflux pump, in human CD8+T cells: a role in cytotoxic effector function. J Clin Immunol. 1992;12:451–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

