Iron, the retina and the lens: a focused review
- PMID: 20230820
- PMCID: PMC2919496
- DOI: 10.1016/j.exer.2010.03.003
Iron, the retina and the lens: a focused review
Abstract
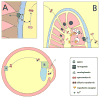
This review is focused on iron metabolism in the retina and in the lens and its relation to their respective age-related pathologies, macular degeneration (AMD) and cataract (ARC). Several aspects of iron homeostasis are considered first in the retina and second in the lens, paying particular attention to the transport of iron through the blood-retinal barrier and through the lens epithelial cell barrier, to the immunochemistry of iron-related proteins and their expression in both the retina and the lens, and to the nature of the photochemical damage caused by UV light on both tissues. A comparative overview of some iron related parameters (total iron, transferrin (Tf), transferrin saturation and total iron binding capacity), in plasma and ocular tissues and fluids of three animal species is also presented. Based on results selected from the literature reviewed, and our own results, a scheme for the overall circulation of iron within and out of the eye is proposed, in which, (i) iron is pumped from the retina to the vitreous body by a ferroportin/ferroxidase-mediated process at the endfeet of Müller cells, (ii) vitreal Tf binds this iron and the complex diffuses towards the lens, (iii) the iron/Tf complex is incorporated into the lens extracellular space probably at the lens equator and moves to the epithelial-fiber interface, (iv) upon interaction with Tf receptors of the apical pole of lens epithelial cells, the iron/Tf complex is endocytosed and iron is exported as Fe(3+) by a ferroportin/ferroxidase-mediated process taking place at the basal pole of the epithelial cells, and (v) Fe(3+) is bound to aqueous humor Tf and drained with the aqueous humor into systemic blood circulation for recycling. The proposed scheme represents an example of close cooperation between the retina and the lens to maintain a constant flow of iron within the eye that provides an adequate supply of iron to ocular tissues and secures the systemic recycling of this element. It does not discount the existence of additional ways for iron to leave the eye through the blood-retinal barrier. In this review both AMD and ARC are recognized as multifactorial diseases with an important photoxidative component, and exhibiting a remarkable similitude of altered local iron metabolism. The epidemiological relationship between ARC and ferropenic anemia is explained on the basis that hepcidin, the hormone responsible for the anemia of chronic inflammation, could paradoxically cause intracellular iron overload in the lens by interfering with the proposed ferroportin/ferroxidase-mediated export of iron at the basal side of the anterior lens epithelium. Other authors have suggested that a similar situation is created in the retina in the case of AMD.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
Iron homeostasis and toxicity in retinal degeneration.Prog Retin Eye Res. 2007 Nov;26(6):649-73. doi: 10.1016/j.preteyeres.2007.07.004. Epub 2007 Aug 11. Prog Retin Eye Res. 2007. PMID: 17921041 Free PMC article. Review.
-
A comparative study of iron-related metabolic parameters in the eye of three animal species.P R Health Sci J. 2007 Dec;26(4):373-83. P R Health Sci J. 2007. PMID: 18246966
-
Iron uptake by cultured lens epithelial cells.Graefes Arch Clin Exp Ophthalmol. 1995 Jun;233(6):354-9. doi: 10.1007/BF00200484. Graefes Arch Clin Exp Ophthalmol. 1995. PMID: 7672622
-
Brain iron homeostasis.Dan Med Bull. 2002 Nov;49(4):279-301. Dan Med Bull. 2002. PMID: 12553165 Review.
-
Prion protein modulates iron transport in the anterior segment: Implications for ocular iron homeostasis and prion transmission.Exp Eye Res. 2018 Oct;175:1-13. doi: 10.1016/j.exer.2018.05.031. Epub 2018 May 31. Exp Eye Res. 2018. PMID: 29859760 Free PMC article.
Cited by
-
Topical nutraceutical Optixcare EH ameliorates experimental ocular oxidative stress in rats.J Ocul Pharmacol Ther. 2014 Sep;30(7):593-602. doi: 10.1089/jop.2014.0016. J Ocul Pharmacol Ther. 2014. PMID: 25188009 Free PMC article.
-
Regulations of Retinal Inflammation: Focusing on Müller Glia.Front Cell Dev Biol. 2022 Apr 27;10:898652. doi: 10.3389/fcell.2022.898652. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35573676 Free PMC article. Review.
-
The Possible Positive Mechanisms of Pirenoxine in Cataract Formation.Int J Mol Sci. 2022 Aug 21;23(16):9431. doi: 10.3390/ijms23169431. Int J Mol Sci. 2022. PMID: 36012695 Free PMC article. Review.
-
Therapeutic potential of iron chelators in retinal vascular diseases.Int J Ophthalmol. 2023 Nov 18;16(11):1899-1910. doi: 10.18240/ijo.2023.11.24. eCollection 2023. Int J Ophthalmol. 2023. PMID: 38028518 Free PMC article. Review.
-
Prions and prion diseases: Insights from the eye.Exp Eye Res. 2020 Oct;199:108200. doi: 10.1016/j.exer.2020.108200. Epub 2020 Aug 25. Exp Eye Res. 2020. PMID: 32858007 Free PMC article. Review.
References
-
- Aisen P. Transferrin Metabolism and the Liver. Semin Liver Dis. 1984;4:193–206. - PubMed
-
- Aisen P. Entry of Iron into Cells: A New Role for the Transferrin Receptor in Modulating Iron Release from Transferrin. Ann Neurol. 1992;32:S62–S68. - PubMed
-
- Aisen P, Enns C, Wessling-Resnick M. Chemistry and biology of eukaryotic iron metabolism. Int J Biochem Cell Biol. 2001:940–959. - PubMed
-
- Amasheh S, Milatz S, Krug SM, Markov AG, Günzel D, Amasheh M, Fromm M. Tight Junction Proteins as Channel Formers and Barrier Builders. Ann N Y Acad Sci. 2009;1165:211–219. - PubMed
-
- Andrews NC, Schmidt PJ. Iron Homeostasis. Annu Rev Physiol. 2007;69:69–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

