Insect ferritins: Typical or atypical?
- PMID: 20230873
- PMCID: PMC2893279
- DOI: 10.1016/j.bbagen.2010.03.004
Insect ferritins: Typical or atypical?
Abstract
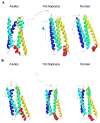
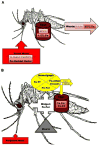
Insects transmit millions of cases of disease each year, and cost millions of dollars in agricultural losses. The control of insect-borne diseases is vital for numerous developing countries, and the management of agricultural insect pests is a very serious business for developed countries. Control methods should target insect-specific traits in order to avoid non-target effects, especially in mammals. Since insect cells have had a billion years of evolutionary divergence from those of vertebrates, they differ in many ways that might be promising for the insect control field-especially, in iron metabolism because current studies have indicated that significant differences exist between insect and mammalian systems. Insect iron metabolism differs from that of vertebrates in the following respects. Insect ferritins have a heavier mass than mammalian ferritins. Unlike their mammalian counterparts, the insect ferritin subunits are often glycosylated and are synthesized with a signal peptide. The crystal structure of insect ferritin also shows a tetrahedral symmetry consisting of 12 heavy chain and 12 light chain subunits in contrast to that of mammalian ferritin that exhibits an octahedral symmetry made of 24 heavy chain and 24 light chain subunits. Insect ferritins associate primarily with the vacuolar system and serve as iron transporters-quite the opposite of the mammalian ferritins, which are mainly cytoplasmic and serve as iron storage proteins. This review will discuss these differences.
Copyright 2010 Elsevier B.V. All rights reserved.
Figures

References
-
- Arosio P, Ingrassia R, Cavadini P. Ferritins: A family of molecules for iron storage, antioxidation and more. Biochim Biophys Acta. 2009;1790:589–599. - PubMed
-
- Leimberg JM, Prus E, Link G, Fibach E, Konijn AM. Iron-chelator complexes as iron sources for early developing human erythroid precursors. Transl Res. 2008;151:88–96. - PubMed
-
- Fisher J, Devraj K, Ingram J, Slagle-Webb B, Madhankumar AB, Liu X, Klinger M, Simpson IA, Connor JR. Ferritin: a novel mechanism for delivery of iron to the brain and other organs. Am J Physiol Cell Physiol. 2007;293:C641–649. - PubMed
-
- Levi S, Arosio P. Mitochondrial ferritin. Int J Biochem Cell Biol. 2004;36:1887–1889. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

