Automated matching software for clinical trials eligibility: measuring efficiency and flexibility
- PMID: 20230913
- PMCID: PMC4387843
- DOI: 10.1016/j.cct.2010.03.005
Automated matching software for clinical trials eligibility: measuring efficiency and flexibility
Abstract
Background: Clinical trials (CT) serve as the media that translates clinical research into standards of care. Low or slow recruitment leads to delays in delivery of new therapies to the public. Determination of eligibility in all patients is one of the most important factors to assure unbiased results from the clinical trials process and represents the first step in addressing the issue of under representation and equal access to clinical trials.
Methods: This is a pilot project evaluating the efficiency, flexibility, and generalizibility of an automated clinical trials eligibility screening tool across 5 different clinical trials and clinical trial scenarios.
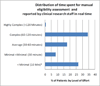
Results: There was a substantial total savings during the study period in research staff time spent in evaluating patients for eligibility ranging from 165h to 1329h. There was a marked enhancement in efficiency with the automated system for all but one study in the pilot. The ratio of mean staff time required per eligible patient identified ranged from 0.8 to 19.4 for the manual versus the automated process.
Conclusion: The results of this study demonstrate that automation offers an opportunity to reduce the burden of the manual processes required for CT eligibility screening and to assure that all patients have an opportunity to be evaluated for participation in clinical trials as appropriate. The automated process greatly reduces the time spent on eligibility screening compared with the traditional manual process by effectively transferring the load of the eligibility assessment process to the computer.
Copyright (c) 2010 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Increasing the efficiency of trial-patient matching: automated clinical trial eligibility pre-screening for pediatric oncology patients.BMC Med Inform Decis Mak. 2015 Apr 14;15:28. doi: 10.1186/s12911-015-0149-3. BMC Med Inform Decis Mak. 2015. PMID: 25881112 Free PMC article.
-
Piloting an automated clinical trial eligibility surveillance and provider alert system based on artificial intelligence and standard data models.BMC Med Res Methodol. 2023 Apr 11;23(1):88. doi: 10.1186/s12874-023-01916-6. BMC Med Res Methodol. 2023. PMID: 37041475 Free PMC article.
-
Computer-based screening of patients with HIV/AIDS for clinical-trial eligibility.Online J Curr Clin Trials. 1995 Mar 28;Doc No 179:[3347 words; 32 paragraphs]. Online J Curr Clin Trials. 1995. PMID: 7719564 Clinical Trial.
-
Artificial Intelligence Tool for Optimizing Eligibility Screening for Clinical Trials in a Large Community Cancer Center.JCO Clin Cancer Inform. 2020 Jan;4:50-59. doi: 10.1200/CCI.19.00079. JCO Clin Cancer Inform. 2020. PMID: 31977254
-
Available, accessible, aware, appropriate, and acceptable: a strategy to improve participation of teenagers and young adults in cancer trials.Lancet Oncol. 2014 Jul;15(8):e341-50. doi: 10.1016/S1470-2045(14)70113-5. Lancet Oncol. 2014. PMID: 24988937 Review.
Cited by
-
Ethical Issues in Social Media Recruitment for Clinical Studies: Ethical Analysis and Framework.J Med Internet Res. 2022 May 3;24(5):e31231. doi: 10.2196/31231. J Med Internet Res. 2022. PMID: 35503247 Free PMC article. Review.
-
Feasibility of feature-based indexing, clustering, and search of clinical trials. A case study of breast cancer trials from ClinicalTrials.gov.Methods Inf Med. 2013;52(5):382-94. doi: 10.3414/ME12-01-0092. Epub 2013 May 13. Methods Inf Med. 2013. PMID: 23666475 Free PMC article.
-
Automatic trial eligibility surveillance based on unstructured clinical data.Int J Med Inform. 2019 Sep;129:13-19. doi: 10.1016/j.ijmedinf.2019.05.018. Epub 2019 May 23. Int J Med Inform. 2019. PMID: 31445247 Free PMC article.
-
Criteria2Query: a natural language interface to clinical databases for cohort definition.J Am Med Inform Assoc. 2019 Apr 1;26(4):294-305. doi: 10.1093/jamia/ocy178. J Am Med Inform Assoc. 2019. PMID: 30753493 Free PMC article.
-
User Requirements for an Electronic Patient Recruitment System: Semistructured Interview Analysis After First Implementation in 3 German University Hospitals.JMIR Hum Factors. 2024 Sep 27;11:e56872. doi: 10.2196/56872. JMIR Hum Factors. 2024. PMID: 39331958 Free PMC article.
References
-
- Ellis P, Butow PN, Tattersall MHN, Dunn SM. Accrual to clinical trials in breast cancer. Annual Scientific Meeting of the Clinical Oncological Society of Australia; Brisbane, Australia. 1996. 1996.
-
- Lara PN, Jr, Higdon R, Lim N, Kwan K, Tanaka M, Lau DH, et al. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J Clin Oncol. 2001;19(6):1728–1733. - PubMed
-
- Avis NE, Smith KW, Link CI, Hortobagyi GN, Rivera N. Factors associated with participation in breast cancer clinical trials. J Clin Oncol. 2006;24(12):1860–1867. - PubMed
-
- Kolata G. Forty years' war: lack of study volunteers hobbles cancer fight. New York Times; 2009. Aug 3,
-
- Winn RJ. Obstacles to the accrual of patients to clinical trials in the community setting. Semin Oncol. 1994;21(4 suppl 7):112–117. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical