Altered phosphorylation of cytoskeleton proteins in sickle red blood cells: the role of protein kinase C, Rac GTPases, and reactive oxygen species
- PMID: 20231105
- PMCID: PMC2878931
- DOI: 10.1016/j.bcmd.2010.02.006
Altered phosphorylation of cytoskeleton proteins in sickle red blood cells: the role of protein kinase C, Rac GTPases, and reactive oxygen species
Abstract
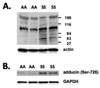
The small Rho GTPases Rac1 and Rac2 regulate actin structures and mediate reactive oxygen species (ROS) production via NADPH oxidase in a variety of cells. We have demonstrated that deficiency of Rac1 and Rac2 GTPases in mice disrupts the normal hexagonal organization of the RBC cytoskeleton and reduces erythrocyte deformability. This is associated with increased phosphorylation of adducin at Ser-724, (corresponding to Ser-726 in human erythrocytes), a domain target of protein kinase C (PKC). PKC phosphorylates adducin and leads to decreased F-actin capping and dissociation of spectrin from actin, implicating a significant role of such phosphorylation in cytoskeletal remodeling. We evaluated adducin phosphorylation in erythrocytes from patients with sickle cell disease and found it consistently increased at Ser-726. In addition, ROS concentration is elevated in sickle erythrocytes by 150-250% compared to erythrocytes from normal control individuals. Here, we review previous studies demonstrating that altered phosphorylation of erythrocyte cytoskeletal proteins and increased ROS production result in disruption of cytoskeleton stability in healthy and sickle cell erythrocytes. We discuss in particular the known and potential roles of protein kinase C and the Rac GTPases in these two processes.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures


References
-
- Nagel RL. Pleiotropic and epistatic effects in sickle cell anemia. Curr Opin Hematol. 2001;8:105–110. - PubMed
-
- Heeney MM, Ware RE. Hydroxyurea for children with sickle cell disease. Pediatr Clin North Am. 2008;55:483–501. x. - PubMed
-
- Ballas SK, Mohandas N. Sickle red cell microrheology and sickle blood rheology. Microcirculation. 2004;11:209–225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

