Endoplasmic reticulum export, subcellular distribution, and fibril formation by Pmel17 require an intact N-terminal domain junction
- PMID: 20231267
- PMCID: PMC2871485
- DOI: 10.1074/jbc.M109.097725
Endoplasmic reticulum export, subcellular distribution, and fibril formation by Pmel17 require an intact N-terminal domain junction
Abstract
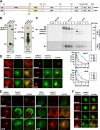
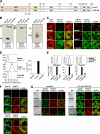
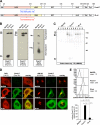
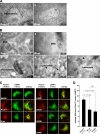
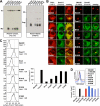
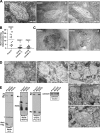
Pmel17 is a melanocyte/melanoma-specific protein that subcellularly localizes to melanosomes, where it forms a fibrillar matrix that serves for the sequestration of potentially toxic reaction intermediates of melanin synthesis and deposition of the pigment. As a key factor in melanosomal biogenesis, understanding intracellular trafficking and processing of Pmel17 is of central importance to comprehend how these organelles are formed, how they mature, and how they function in the cell. Using a series of deletion and missense mutants of Pmel17, we are able to show that the integrity of the junction between the N-terminal region and the polycystic kidney disease-like domain is highly crucial for endoplasmic reticulum export, subcellular targeting, and fibril formation by Pmel17 and thus for establishing functional melanosomes.
Figures

References
-
- Lee Z. H., Hou L., Moellmann G., Kuklinska E., Antol K., Fraser M., Halaban R., Kwon B. S. (1996) J. Invest. Dermatol. 106, 605–610 - PubMed
-
- Quevedo W. C., Fleischmann R. D., Dyckman J. (1981) in Phenotypic Expression in Pigment Cells (Seiji M. ed) pp. 177–184, Tokyo University Press, Tokyo
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

