Platelet-derived growth factor-DD targeting arrests pathological angiogenesis by modulating glycogen synthase kinase-3beta phosphorylation
- PMID: 20231273
- PMCID: PMC2865282
- DOI: 10.1074/jbc.M110.113787
Platelet-derived growth factor-DD targeting arrests pathological angiogenesis by modulating glycogen synthase kinase-3beta phosphorylation
Abstract
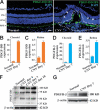
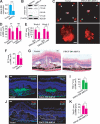
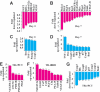
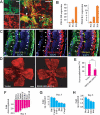
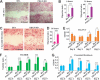
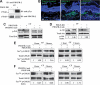
Platelet-derived growth factor-DD (PDGF-DD) is a recently discovered member of the PDGF family. The role of PDGF-DD in pathological angiogenesis and the underlying cellular and molecular mechanisms remain largely unexplored. In this study, using different animal models, we showed that PDGF-DD expression was up-regulated during pathological angiogenesis, and inhibition of PDGF-DD suppressed both choroidal and retinal neovascularization. We also demonstrated a novel mechanism mediating the function of PDGF-DD. PDGF-DD induced glycogen synthase kinase-3beta (GSK3beta) Ser(9) phosphorylation and Tyr(216) dephosphorylation in vitro and in vivo, leading to increased cell survival. Consistently, GSK3beta activity was required for the antiangiogenic effect of PDGF-DD targeting. Moreover, PDGF-DD regulated the expression of GSK3beta and many other genes important for angiogenesis and apoptosis. Thus, we identified PDGF-DD as an important target gene for antiangiogenic therapy due to its pleiotropic effects on vascular and non-vascular cells. PDGF-DD inhibition may offer new therapeutic options to treat neovascular diseases.
Figures



Similar articles
-
PDGF-CC blockade inhibits pathological angiogenesis by acting on multiple cellular and molecular targets.Proc Natl Acad Sci U S A. 2010 Jul 6;107(27):12216-21. doi: 10.1073/pnas.1004143107. Epub 2010 Jun 21. Proc Natl Acad Sci U S A. 2010. PMID: 20566880 Free PMC article.
-
Survival effect of PDGF-CC rescues neurons from apoptosis in both brain and retina by regulating GSK3beta phosphorylation.J Exp Med. 2010 Apr 12;207(4):867-80. doi: 10.1084/jem.20091704. Epub 2010 Mar 15. J Exp Med. 2010. PMID: 20231377 Free PMC article.
-
Platelet-derived growth factor CC-mediated neuroprotection against HIV Tat involves TRPC-mediated inactivation of GSK 3beta.PLoS One. 2012;7(10):e47572. doi: 10.1371/journal.pone.0047572. Epub 2012 Oct 15. PLoS One. 2012. PMID: 23077641 Free PMC article.
-
PDGF-DD, a novel mediator of smooth muscle cell phenotypic modulation, is upregulated in endothelial cells exposed to atherosclerosis-prone flow patterns.Am J Physiol Heart Circ Physiol. 2009 Feb;296(2):H442-52. doi: 10.1152/ajpheart.00165.2008. Epub 2008 Nov 21. Am J Physiol Heart Circ Physiol. 2009. PMID: 19028801 Free PMC article.
-
PDGF-C and PDGF-D signaling in vascular diseases and animal models.Mol Aspects Med. 2018 Aug;62:1-11. doi: 10.1016/j.mam.2018.01.005. Epub 2018 Feb 14. Mol Aspects Med. 2018. PMID: 29410092 Review.
Cited by
-
Integrative analysis of miRNA and mRNA paired expression profiling of primary fibroblast derived from diabetic foot ulcers reveals multiple impaired cellular functions.Wound Repair Regen. 2016 Nov;24(6):943-953. doi: 10.1111/wrr.12470. Epub 2016 Oct 4. Wound Repair Regen. 2016. PMID: 27607190 Free PMC article.
-
PDGFD switches on stem cell endothelial commitment.Angiogenesis. 2022 Nov;25(4):517-533. doi: 10.1007/s10456-022-09847-4. Epub 2022 Jul 20. Angiogenesis. 2022. PMID: 35859222 Free PMC article.
-
A first-in-human phase I dose-escalation, pharmacokinetic, and pharmacodynamic evaluation of intravenous LY2090314, a glycogen synthase kinase 3 inhibitor, administered in combination with pemetrexed and carboplatin.Invest New Drugs. 2015 Dec;33(6):1187-96. doi: 10.1007/s10637-015-0278-7. Epub 2015 Sep 25. Invest New Drugs. 2015. PMID: 26403509 Clinical Trial.
-
Platelet-derived growth factor alpha and beta receptors have overlapping functional activities towards fibroblasts.Fibrogenesis Tissue Repair. 2013 May 10;6(1):10. doi: 10.1186/1755-1536-6-10. Fibrogenesis Tissue Repair. 2013. PMID: 23663505 Free PMC article.
-
Canonical Wnt suppressor, Axin2, promotes colon carcinoma oncogenic activity.Proc Natl Acad Sci U S A. 2012 Jul 10;109(28):11312-7. doi: 10.1073/pnas.1203015109. Epub 2012 Jun 27. Proc Natl Acad Sci U S A. 2012. PMID: 22745173 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

