Incorporation of tyrosine and glutamine residues into the soluble guanylate cyclase heme distal pocket alters NO and O2 binding
- PMID: 20231286
- PMCID: PMC2878511
- DOI: 10.1074/jbc.M109.098269
Incorporation of tyrosine and glutamine residues into the soluble guanylate cyclase heme distal pocket alters NO and O2 binding
Abstract
Nitric oxide (NO) is the physiologically relevant activator of the mammalian hemoprotein soluble guanylate cyclase (sGC). The heme cofactor of alpha1beta1 sGC has a high affinity for NO but has never been observed to form a complex with oxygen. Introduction of a key tyrosine residue in the sGC heme binding domain beta1(1-385) is sufficient to produce an oxygen-binding protein, but this mutation in the full-length enzyme did not alter oxygen affinity. To evaluate ligand binding specificity in full-length sGC we mutated several conserved distal heme pocket residues (beta1 Val-5, Phe-74, Ile-145, and Ile-149) to introduce a hydrogen bond donor in proximity to the heme ligand. We found that the NO coordination state, NO dissociation, and enzyme activation were significantly affected by the presence of a tyrosine in the distal heme pocket; however, the stability of the reduced porphyrin and the proteins affinity for oxygen were unaltered. Recently, an atypical sGC from Drosophila, Gyc-88E, was shown to form a stable complex with oxygen. Sequence analysis of this protein identified two residues in the predicted heme pocket (tyrosine and glutamine) that may function to stabilize oxygen binding in the atypical cyclase. The introduction of these residues into the rat beta1 distal heme pocket (Ile-145 --> Tyr and Ile-149 --> Gln) resulted in an sGC construct that oxidized via an intermediate with an absorbance maximum at 417 nm. This absorbance maximum is consistent with globin Fe(II)-O(2) complexes and is likely the first observation of a Fe(II)-O(2) complex in the full-length alpha1beta1 protein. Additionally, these data suggest that atypical sGCs stabilize O(2) binding by a hydrogen bonding network involving tyrosine and glutamine.
Figures



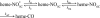



Similar articles
-
Probing domain interactions in soluble guanylate cyclase.Biochemistry. 2011 May 24;50(20):4281-90. doi: 10.1021/bi200341b. Epub 2011 May 3. Biochemistry. 2011. PMID: 21491957 Free PMC article.
-
Insights into the distal heme pocket of H-NOX using fluoride as a probe for H-bonding interactions.J Inorg Biochem. 2013 Sep;126:91-5. doi: 10.1016/j.jinorgbio.2013.05.012. Epub 2013 Jun 3. J Inorg Biochem. 2013. PMID: 23792914
-
Ligand selectivity of soluble guanylyl cyclase: effect of the hydrogen-bonding tyrosine in the distal heme pocket on binding of oxygen, nitric oxide, and carbon monoxide.J Biol Chem. 2006 Sep 22;281(38):27836-45. doi: 10.1074/jbc.M601078200. Epub 2006 Jul 24. J Biol Chem. 2006. PMID: 16864588
-
How do heme-protein sensors exclude oxygen? Lessons learned from cytochrome c', Nostoc puntiforme heme nitric oxide/oxygen-binding domain, and soluble guanylyl cyclase.Antioxid Redox Signal. 2012 Nov 1;17(9):1246-63. doi: 10.1089/ars.2012.4564. Epub 2012 Apr 10. Antioxid Redox Signal. 2012. PMID: 22356101 Free PMC article. Review.
-
Ligand discrimination in soluble guanylate cyclase and the H-NOX family of heme sensor proteins.Curr Opin Chem Biol. 2005 Oct;9(5):441-6. doi: 10.1016/j.cbpa.2005.08.015. Curr Opin Chem Biol. 2005. PMID: 16125437 Review.
Cited by
-
Quaternary structure controls ligand dynamics in soluble guanylate cyclase.J Biol Chem. 2012 Feb 24;287(9):6851-9. doi: 10.1074/jbc.M111.299297. Epub 2012 Jan 4. J Biol Chem. 2012. PMID: 22223482 Free PMC article.
-
Controlling a burn: outer-sphere gating of hydroxylamine oxidation by a distal base in cytochrome P460.Chem Sci. 2019 Mar 6;10(13):3756-3764. doi: 10.1039/c9sc00195f. eCollection 2019 Apr 7. Chem Sci. 2019. PMID: 31015919 Free PMC article.
-
Controlling conformational flexibility of an O₂-binding H-NOX domain.Biochemistry. 2011 Aug 16;50(32):6832-40. doi: 10.1021/bi200788x. Epub 2011 Jul 15. Biochemistry. 2011. PMID: 21721586 Free PMC article.
-
Oxygen binding and redox properties of the heme in soluble guanylate cyclase: implications for the mechanism of ligand discrimination.J Biol Chem. 2011 May 6;286(18):15678-87. doi: 10.1074/jbc.M110.177576. Epub 2011 Mar 8. J Biol Chem. 2011. PMID: 21385878 Free PMC article.
-
Probing domain interactions in soluble guanylate cyclase.Biochemistry. 2011 May 24;50(20):4281-90. doi: 10.1021/bi200341b. Epub 2011 May 3. Biochemistry. 2011. PMID: 21491957 Free PMC article.
References
-
- Münzel T., Feil R., Mülsch A., Lohmann S. M., Hofmann F., Walter U. (2003) Circulation 108, 2172–2183 - PubMed
-
- Sanders K. M., Ward S. M., Thornbury K. D., Dalziel H. H., Westfall D. P., Carl A. (1992) Jpn. J. Pharmacol. 58, P220–P225 - PubMed
-
- Warner T. D., Mitchell J. A., Sheng H., Murad F. (1994) Adv. Pharmacol. 26, 171–194 - PubMed
-
- Vermeersch P., Buys E., Pokreisz P., Marsboom G., Ichinose F., Sips P., Pellens M., Gillijns H., Swinnen M., Graveline A., Collen D., Dewerchin M., Brouckaert P., Bloch K. D., Janssens S. (2007) Circulation 116, 936–943 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

